Small animal - March 2020
Emergency cytology II - cytology of fluids
In part two of this emergency cytology focus, Poppy Gant BVSc MRCVS looks at the preparation and assessment of cytology from various fluids, including urine, body cavity effusions and airway lavages. Examples of emergency presentations in which immediate cytological assessment is useful will concurrently be discussed.
Obtaining samples
Fluid samples should always be obtained in a sterile manner to reduce the risk of contamination. A common reason for cytological assessment in the emergency setting is to confirm bacterial sepsis and contamination can make interpretation difficult.
Samples should always be distributed immediately into ethylenediaminetetraacetic acid (EDTA [for cytology]) and plain (for culture) tubes and stored in the fridge. Fresh, air-dried smears should also be made as soon as possible to minimise any aging changes that can occur if samples are not immediately analysed.
The benefit and risk of obtaining samples in emergency patients should always be considered, particularly for dyspnoeic patients, in which a sedation or general anaesthesia can be particularly risky. However, in general, the majority of sampling procedures can either be performed conscious or with minimal sedation, including butorphanol or pure mu opioids alone, or with the addition of benzodiazepines.
Point-of-care ultrasound is becoming more frequently utilised and can be invaluable in obtaining small volume samples via guided thoracocentesis or abdominocentesis or obtaining urine in patients where blind cystocentesis is not possible.
Both peritoneal and pleural effusions are most easily sampled using a butterfly needle and three-way tap connected to a syringe. Particularly for pleural effusions, this allows easy therapeutic, as well as diagnostic draining. It is less likely that abdominal fluid will require therapeutic draining, unless the volume is compromising the patient's breathing.
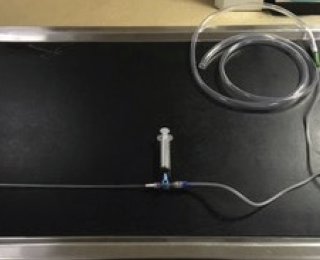
Endotracheal or trans-tracheal lavages can either be performed manually, or the yield can be significantly increased using the equipment noted in figure 2. Transtracheal lavage is only suitable for larger dogs but endotracheal lavage can easily be performed in all patients. Although guided bronchoalveolar lavage is the gold standard for obtaining lower airway samples, dyspnoeic patients may not be stable for this procedure. However, a blind sample can often provide sufficient information to guide treatment and can be performed much more quickly.
Equipment required
- Sterile urinary catheter to deliver saline and aspirate fluid
- Sterile cuffed endotracheal tubes (ETT)
- A sterile 10-20ml syringe with aliquots of 0.9% sterile saline
- Three-way tap
- A sterile 60ml syringe for suctioning
Additional equipment for suction-assisted lavage
- Suction cannister
- Tubing to connect to suction machine
- Male and female Luer lock connectors and tapered tubing adaptors
Procedure for endotracheal lavage
- Pre-oxygenate the patient
- Administer fast-acting sedation, eg. propofol (ideally with fentanyl or midazolam to reduce the dose required)
- Intubate with sterile endotracheal tube, minimising oropharyngeal contamination as much as possible
- Insert a sterile urinary catheter down the endotracheal tube. If using suction, ensure tap is ‘off’ to suction machine
- Instil 5 ml of sterile saline for cats and small dogs and 10ml for larger dogs. Coupage can be performed concurrently to loosen secretions and increase yield
- If using suction, then turn tap to open urinary catheter to suction. If suction is not available, then manually aspirate using 60ml syringe
- Typically, only a small amount of fluid is obtained. The process can be repeated again if required following another period of pre-oxygenation
How to prepare a smear for cytology
This is very similar to making a blood smear but with some slight adjustments depending on the viscosity of the sample and the expected cellularity.
1. Use a room temperature EDTA sample.
2. For samples expected to be of low cellularity, centrifuge the sample on a slow setting for two minutes. Viscous samples often do not need to be centrifuged.
- If centrifuged, then remove the supernatant and resuspend the sediment
3. Use a microhaematocrit tube to place a small amount of sample at the base of a slide.
4. Hold spreader slide at 45o (or more if sample not very viscous).
5. Back into the drop of fluid and as soon as the drop spreads along the edge, push forward in a smooth, reasonably fast motion
6. If slides are expected to be poorly cellular, you can also make a ‘line preparation’.
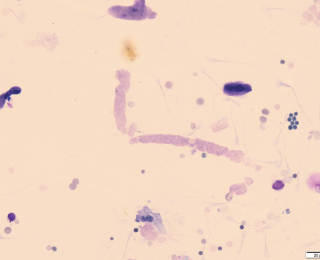
- This means stopping the slide before generating a ‘feathered edge’. This often gives a line of concentrated white blood cells (and other heavier fluid constituents) (see figure 3).
7. Apply only enough pressure to keep the spreader slide on the glass.
8. Allow slide to air dry.
9. Stain with rapid commercial Romanowsky stains (eg. Diff-Quik or equivalent). Generally, 10-15 ‘agitations’ in each solution, blotting in between, then rinsing with distilled water and again allowing to air dry).
This process can be used for all fluid samples. The exception to this would be when analysing a ‘wet’ preparation for urine cytology, which is required to assess for urine casts and crystals which can be damaged during centrifuging. For this, a single drop of urine is placed on the centre of a slide and a cover slip placed on top.
Urine
What questions can be quickly answered in the emergency setting?
- Is there evidence of bacterial sepsis?
- Is there evidence of a particular type of crystalluria?
- Is there evidence of urinary casts?
Urinary tract infection
Samples obtained via cystocentesis should not have any bacteria and are therefore best for documenting the presence of a urinary tract infection. Samples obtained by urinary catheter or free catch methods may still have extracellular bacteria from the lower urinary tract. However, the presence of intracellular bacteria in these samples does suggest an active infection and warrants cystocentesis to obtain a sample for culture and sensitivity depending on the presentation. Some patients eg. diabetics, may not mobilise their neutrophils and may still have a bacterial infection, despite a lack of sediment. A urine culture is therefore still important in these patients.
Crystalluria
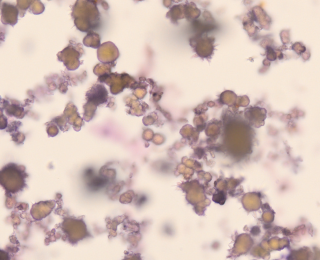
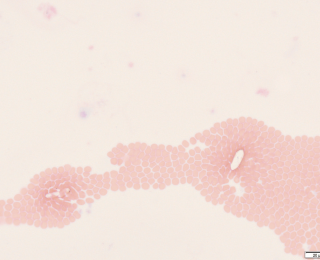
It is important to assess for crystals on a fresh, un-centrifuged sample. Fresh, unrefrigerated urine samples should generally have no, or very few, crystals. However, stored urine, especially if super saturated, will often have crystals present. In the emergency setting, identifying crystals is important if animals are presenting for lower urinary tract signs, such as urethral obstruction, as this may be relevant for their ongoing management. For example, documenting urate stones means the patient should be investigated for portosystemic shunting (Figure 5). These stones can also be seen in puppies presenting for emergency management of hepatic encephalopathy secondary to congenital portosystemic shunting.
The other important presentation that is not to be missed, is ethylene glycol toxicity, in which patients can eventually develop calcium oxalate monohydrate ‘picket fence’ crystals (Figure 6). However, this can also be seen in the urine of patients with hypercalciuria from other causes eg. paraneoplastic hypercalcaemia with lymphoma and are therefore still not 100% sensitive.
Urinary casts
Casts are cylindrical structures with parallel sides made up of clumps of cells or other material in a protein matrix. They generally form during renal tubular damage. Accurate identification of constituents is often difficult because casts are rarely formed of only one element and can include cells and debris, protein and fat. Casts are fragile and may be damaged with centrifuging, so it is best to look for them initially on a fresh sample with cover slip.
Additional advice for urine cytology
‘Sedi-stain’ is still frequently used in general practice. However, it is prone to developing precipitates and, less commonly, bacterial contamination. This makes assessing for urinary sepsis more difficult. It is usually preferable to instead make a dry smear and stain with ‘Diff-Quik’.
Pleural and peritoneal effusion cytology
What questions can be quickly answered in the emergency setting?
- Is there any evidence of sepsis, eg. pyothorax or septic peritonitis?
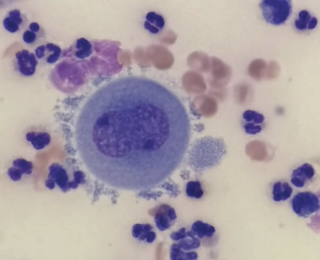
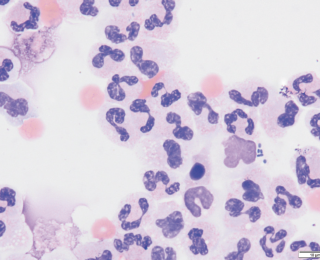
Many effusions can grossly appear very suspicious for septic peritonitis due to high levels of inflammation. However, there are causes of exudative effusions eg. pancreatitis that can still be sterile (Figure 8). The presence of effusions themselves is also enough to stimulate an inflammatory reaction. It is important to be aware that this often leads to the presence of reactive mesothelial cells, especially in pleural effusions (Figure 9). These highly reactive cells often look neoplastic, but this cannot be determined from cytology alone and requires histopathology.
Assessing for sepsis
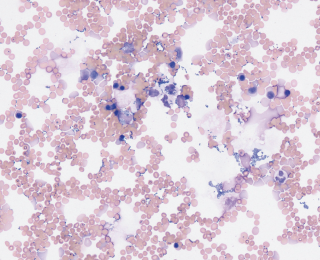
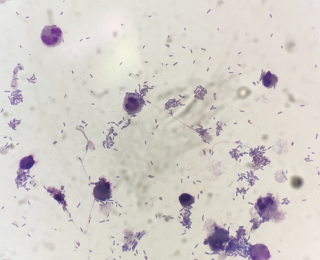
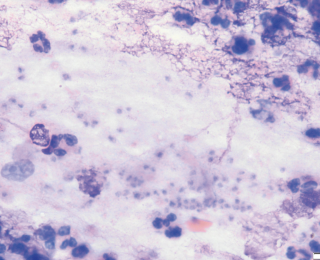
Depending on the underlying pathology, bacteria in effusions can be very variable in number. For example, a dog with a septic peritonitis secondary to foreign body ingestion and severe small intestinal perforation can have large numbers of bacteria on cytology (Figure 10). However, if the same patient undergoes enterotomy and is placed on antimicrobials but two days later develops a pyrexia and increase in peritoneal effusion, often it can be very difficult to find bacteria as numbers are likely to be very low (Figure 11).
In these situations, often the sample must be processed using a special centrifuge that is designed to evenly deposit cells in a monolayer onto a glass slide (Cytospin). Absence of sepsis should therefore not be based on the inability to find bacteria using manual cytology.
Additional advice for effusion cytology
It is useful to make two smears – one in which cells are evenly spread but also a line preparation. Depending on the sample cellularity, either slide may be more useful in assessing for abnormal cells or sepsis.
Lavages of the lower respiratory tract
What questions can be quickly answered in the emergency setting?
- Is there any evidence of sepsis?
- What is the most likely causative agent?
- If no sepsis, is there evidence of inflammation?
The majority of infections will likely be bacterial (Figure 12). Bacteria may be primary or secondary pathogens and the signalment and history will likely help determine this. For example, dogs with acute onset respiratory signs after recent vomiting are more likely to have aspirated gastrointestinal contents whereas dogs with chronic intermittent respiratory disease may be more likely to have an underlying disease with secondary opportunistic infections. It is also possible to see evidence of fungal infections, although they are much less common. Once example is Pneumocystis (Figure 13), which can cause life threatening dyspnoea. This is particularly seen in Cavalier King Charles Spaniels who have an underlying immunodeficiency that predisposes them to infection.
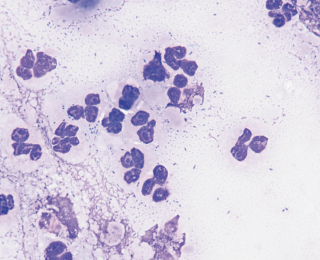
Severe, non-septic inflammatory lung disease can also result in an emergency presentation. One particularly important example is eosinophilic bronchopneumopathy. This typically occurs in younger dogs who may or may not have a history of coughing. Performing endotracheal lavage in these patients and documenting large numbers of eosinophils allows steroid medication to be introduced immediately.
Additional advice for lavage cytology
It is important to be aware that contamination from the upper respiratory tract can easily occur. Several features may help differentiate a contamination from a true infection:
Finding intracellular bacteria is more likely associated with active infection.
Depending on the signalment and history, a mixed bacterial sepsis may more likely be a contaminant.
The presence of Simonsiella (large, stacked, rod-shaped bacteria) are normal inhabitants of the oral cavity and help support oropharyngeal contamination.
If bacteria are noted on cytology, then always submit for culture and sensitivity testing.
Depending on the case, additional testing may be requested on this fluid, including Mycoplasma or Bordetella bronchiseptica PCR.
Summary
Although emergency cytology may take a little more preparation, cytological assessment can still be rapidly performed and, in many circumstances, can directly alter the continued diagnostics and treatment plan for a patient. In the most severely affected patients, this can even make the difference to their overall outcome.
Sincere thanks go to Dr Laureen Peters DVM MVetMed, MRCVS ACVP and Paul Barber (senior haematology technician) for their assistance in providing photomicrographs
References available on request.
1. What is the best way to preserve samples for later cytological assessment?
a. Put samples in the freezer
B. Store in EDTA tube
C. Make fresh, air dried smears
D. Centrifuge samples and remove the supernatant
2. Which crystals are associated with ethylene glycol toxicity?
a. Calcium oxalate monohydrate
B. Calcium oxalate dihydrate
C. Calcium biurate
D. Calcium carbonate
3. Simonsiella bacteria found in an endotracheal wash are most likely to represent what?
a. Oropharyngeal contamination
B. Chronic pulmonary disease
C. Poorly sterilised endotracheal tube
D. Aspiration of gastric contents
4. What does the presence of casts in the urine suggest?
a. Chronic glomerular disease
B. Acute tubular injury
C. Supersaturated urine
D. Post-obstructive diuresis
5. Which cells from peritoneal and pleural effusions can frequently display criteria of malignancy in patients without neoplasia?
a. Lymphocytes
B. Macrophages
C. Fibroblasts
D. Mesothelial cells