Testing for Johne’s disease in Ireland
Testing is one of the essential requirements of the Irish Johne’s Control Programme (IJCP), but is nuanced in terms of identification of animals to be tested, sampling, and interpretation of results. Lawrence Gavey BVSc, BSc (Hons), Johne’s Disease Programme Manager at Animal Health Ireland, discusses testing as it applies to the IJCP
The IJCP (Gavey et al, 2021) is a voluntary programme widely supported by dairy and beef industry stakeholders. It provides funded supports for the control of spread of Mycobacterium avium subsp. paratuberculosis (MAP), the causative organism of Johne’s disease (JD).
As at March 1, 2022, there were 1,968 Irish cattle herds (1,951 dairy, 17 beef) registered in the programme.
The IJCP has four objectives:
1. Enhance the ability of participating farmers to keep their herds clear of JD.
2. Assist participating farmers to reduce the level of infection in their herds, where present.
3. Provide additional reassurance to the marketplace in relation to Ireland’s efforts to control JD.
4. Improve calf health and farm biosecurity in participating farms.
These objectives are relevant to the long-term economic sustainability of herds and the Irish dairy industry, and will contribute to achieving animal health and welfare and greenhouse gas targets. The objectives do not include eradication of infection.
There are four programme activities required of participating farms:
1. Annual veterinary risk assessment and management plan (VRAMP) to guide reducing spread of infection between and within herds.
2. Annual whole herd testing by ELISA on milk or blood samples from all cattle aged two years or more (eligible animals).
3. Ancillary testing by PCR or culture of faecal samples from animals with non-negative ELISA results, unless the herd has prior confirmation of infection.
4. Detailed epidemiological investigation of herds with confirmed infection through a TASAH activity.
These activities are summarised in the programme flowchart (Figure 1, details at: https://animalhealthireland.ie/resources/?resource_type[]=documents&prog[]=johnes-disease)
Testing under the IJCP has three roles:
1. Identify herds infected with MAP – the estimated herd prevalence of JD in the Irish dairy industry is 30 per cent (McAloon et al, 2016), so 70 per cent of herds are likely not infected. Testing can detect infection before clinical disease becomes apparent.
2. Detect infected animals – to inform epidemiological analysis of the herd and prioritise VRAMP recommendations and other control actions.
3. Assurance – consistent negative results offer assurance of low-risk status, to guide bioexclusion and to support marketing opportunities for low-risk stock.
All testing is conducted by designated laboratories, listed on the AHI website. This is essential to maintain testing standards and for uploading test results to the programme database provided by ICBF. These results are then visible to herd owners and AVPs on the IJCP dashboard (Figures 2a & b).
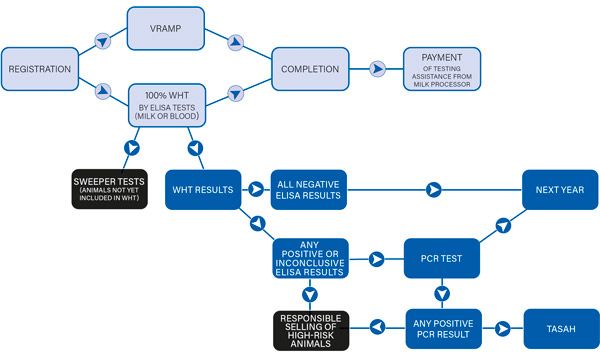
Figure 1. Programme flowchart.
Approved veterinary practitioners (AVPs), trained by the programme, conduct VRAMPs and provide or support testing and interpretation of results.
Samples should be submitted immediately to the laboratory, to minimise discrepancy between sampling and test dates as recorded on ICBF. To alleviate this discrepancy, the programme will include sampling date on the submission form and on ICBF screens.
AVPs receive copies of blood-ELISA and faecal PCR test results directly from the laboratory and should monitor their ICBF Johne’s dashboard and SMS messages from AHI to be aware of milk-ELISA results.
Limitations of Johne’s testing
MAP ELISA testing aims to detect MAP-specific antibodies, raised by the host animal’s immune system in response to MAP infection. Faecal PCR testing for MAP aims to detect MAP-specific fragments (typically IS900) of DNA. The antibody or DNA fragment must be present in the sample above the level of detection for a test result to be positive.
There are well-recognised limitations to Johne’s testing, due to the nature of MAP and the animals’ immune responses, rather than deficiencies in the tests themselves. MAP is characterised by slow growth, both in the animal and in the laboratory, and a waxy coat and intra-cellular habit that protect it against recognition and specific humoral response by the host’s immune system. JD typically has a prolonged latent period (years), between infection and the onset of faecal shedding and development of an antibody response. Production of detectable MAP-specific antibodies or DNA, as an animal’s condition progresses from infected towards infectious and affected, rarely occurs within two years of infection.
Approved veterinary practitioners for the IJCP must understand these limitations to accurately interpret test results and advise clients.
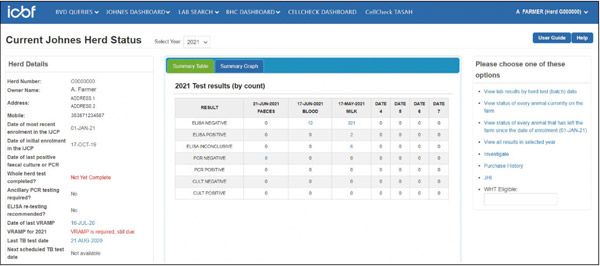
Figure 2a. ICBF Johne’s herd screen.
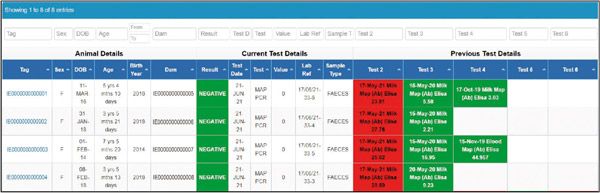
Figure 2b. ICBF screen showing tested animals.
Sensitivity and specificity (Loong, 2003)
In the context of MAP testing, the terms sensitivity and specificity refer to the ability of the test to correctly identify whether an animal is or is not infected.
Sensitivity – probability that the test will correctly detect infected animals (a sensitive test will have no false negatives; 100 per cent sensitivity).
Specificity – probability that the test will correctly identify animals that are not infected (a specific test will have no false positives; 100 per cent specificity).
For MAP testing, the ELISA test has low sensitivity (McAloon et al, 2020), in the order of 15 per cent. Therefore, overall, only 15 per cent of MAP-infected animals will test positive due to absence (or below limit of detection) of antibodies in 85 per cent of infected animals. Sensitivity increases as disease progresses (Nielsen and Toft, 2008) and, therefore, is much lower in younger age cohorts, approaching zero in calves (although maternally-derived antibodies may be detected).
Similarly for PCR testing, shedding of MAP in faeces typically does not occur for several years after infection.
However, MAP testing has high specificity (Nielsen and Toft, 2008). MAP ELISA test kits claim to have specificities of 96 to 98 per cent, so that a high proportion of positive ELISA results will be due to the animal being infected, while two to four per cent of positive ELISA results may be false positives due to the test reacting to a sample component other than MAP-specific antibody. Ancillary faecal testing (by PCR or culture) is determined to have a specificity of 1.0; all positive results are due to infection being present in the herd.
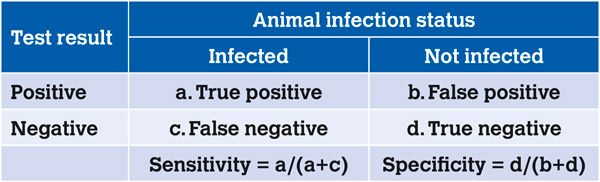
Table 1. Sensitivity and specificity.
Interpretations
Taking the above into account, the IJCP has specific requirements and recommendations, as developed by the Technical Working Group:
Animals aged less than two years should not be tested by MAP-ELISA; the test has a very low sensitivity for this group, so it is unlikely to detect infected animals and any positive results are likely to be false.
Due to low test sensitivity, a negative test result for an individual animal should not be used for assurance, e.g., a pre- or post-movement test to determine the animal’s status.
Despite the low sensitivity of the ELISA test at the individual animal level, the ELISA has value when applied as a herd test. Testing a relatively large number of animals with negative results provides confidence that the herd is not infected, with this growing with repeated rounds of negative testing. This assessment can inform management priorities and risk-based assurance for trading of animals from the herd.
Although a positive ELISA result in a herd that has already been confirmed to be infected by PCR may technically be a true or a false positive, almost all positive ELISA results will be true positives (due to the high specificity of the ELISA test); and the animal with a positive ELISA test result can be confidently interpreted as being infected. This is the reasoning for the programme not to support or fund ancillary PCR testing of such an animal. (See next paragraph for exceptions.)
The programme recommends not testing within 90 days after a TB skin test, or within seven days after calving (milk sample only), to reduce the incidence of non-negative ELISA results due to non-MAP stimuli (Varges, 2009). Animals with non-negative ELISA results from those periods should be re-tested by ELISA.
MAP-ELISA testing of milk samples is best conducted in mid-lactation. There are relatively more positive and particularly inconclusive ELISA results from milk-ELISA than blood-ELISA testing, particularly in the first week of lactation and the last two months of lactation (Lombard et al, 2006). These higher numbers of positive and inconclusive results for milk testing appear to be due to reduced specificity; that is, it appears that some milk component is causing false-positive results (Nielsen and Toft, 2012).
Confidence in an assessment of low-risk of infection due to a negative whole herd test increases with size of herd and with repeated negative herd tests over time.
Herd testing cannot definitively demonstrate that a herd is ‘free’ of MAP, so the IJCP refers to levels of risk of infection and confidence of freedom rather than absolute freedom.
The higher the prevalence of infection in a herd, the greater the chance of detecting infection through a herd test, e.g., for a herd with only one infected animal, the probability of a false negative result for that animal and thus the herd in a single herd test = 1.0 – 0.15 = 0.85; whereas for a herd with five infected animals, the probability of a false negative result = (1 – 0.15)5 = 0.44
The sensitivity of ELISA testing using blood samples is marginally better than from milk samples but, when spread over a whole herd and for repeated herd tests and taking into account the reduced cost and better convenience of milk-ELISA testing, there is little practical difference (Sergeant et al, 2019).
Ancillary faecal testing (PCR or culture) is used by the programme to confirm infection in a herd following positive or inconclusive ELISA results. A positive ancillary test result is confirmation of the presence of MAP in the herd, but a negative result does not necessarily mean the animal is not infected.
An animal with a test history of a non-negative ELISA result followed by a negative PCR result may be infected but not yet shedding at a detectable level (pre-shedding, intermittent, low-level etc.), or the ELISA result may be non-specific. Such animals are considered by the programme to be suspect, and they remain suspect unless they have a subsequent ELISA test with a negative result.
Reported ELISA S/P values are not a direct measure of infection, but are indicative of the animal’s immune response to infection. S/P values of an individual animal can fluctuate substantially, especially in the early and late stages of progression of infection. Patterns of ELISA results that are highly suggestive of infection are: repeated positive results; progressively increasing S/P values and/or high S/P values for any individual animal; and many animals in the herd having positive results (high apparent prevalence).
Some animals may be infected and even shed MAP, without ever being ELISA test positive.
Animal movements into a herd are the greatest risk for introduction of infection, but detection of that introduction and/or spread into and within the recipient herd may take many years of herd testing.
Positive results, whether to ELISA or ancillary tests, identify animals at high risk of being infected and/or spreading infection. The IJCP offers a range of management interventions to reduce the risk of spread of infection from these animals, such as isolation from the herd, separation from the rest of the herd at calving, culling, not keeping calves from high-risk cows, and exclusion of their milk and colostrum from feeding replacement calves. Any consideration of these interventions should take into account the context of the farm: clinical disease, herd hygiene, animal movements, farmer’s capacity and priorities towards control of JD.
Sample Scenario
Herd size of 110 cows
True prevalence of 10 per cent = 11 infected cows
0.15 ELISA sensitivity = 2 cows sero-positive
0.25 PCR sensitivity = 0.5 cows PCR positive
0.98 ELISA specificity = 2 cows false positive sero-positive
Summary for typical IJCP herd with infection: Small number of positive ELISA results, unlikely to detect infection with a positive PCR result in the first round of testing. But apparent prevalence and S/P values may be indicative.
Sound VRAMP bio-containment measures are essential to control spread of MAP, irrespective of test outcomes.
Gavey L., Citer L., More S.J. and Graham D. The Irish Johne’s Control Programme. Frontiers of Veterinary Science 2021; 8:703843. doi: 10.3389/fvets.2021.703843
Lombard J.E., Byrem T.M., Wagner B.A., McCluskey B.J. Comparison of Milk and Serum Enzyme–Linked Immunosorbent Assays for Diagnosis of Mycobacterium Avium Subspecies Paratuberculosis Infection in Dairy Cattle. Journal of Veterinary Diagnostic Investigation 2006;18(5): 448-458. doi:10.1177/104063870601800504
Loong T.W. Understanding sensitivity and specificity with the right side of the brain. British Medical Journal 2003 Sep 27; 327(7417): 716-9. doi: 10.1136/bmj.327.7417.716
McAloon C.G., Doherty M.L., Whyte P., O’Grady L., More S.J., Messam L.L.M. et al. Bayesian estimation of prevalence of paratuberculosis in dairy herds enrolled in a voluntary Johne’s Disease Control Programme in Ireland. Preventative Veterinary Medicine 2016; 128:95–100. doi: 10.1016/j.prevetmed.2016.4.014
McAloon C.G., O'Grady L., Botaro B., More S.J., Doherty M., Whyte P., Nielsen S.S., Citer L., Kenny K., Graham D., Green M. Individual and herd-level milk ELISA test status for Johne's disease in Ireland after correcting for non-disease-associated variables. Journal of Dairy Science 2020; 103 (10): 9345-9354. https://doi.org/10.3168/jds.2019-18018.
Nielsen S.S., Toft N. Ante mortem diagnosis of paratuberculosis: a review of accuracies of ELISA, interferon-γ assay and faecal culture techniques. Veterinary Microbiology 2008; 129: 217-235 https://doi.org/10.1016/j.vetmic.2007.12.011
Nielsen S.S., Toft N. Effect of days in milk and milk yield on testing positive in milk antibody ELISA to Mycobacterium avium ssp. paratuberculosis in dairy cattle. Veterinary Immunology and Immunopathology 2012; 149: 6-10. https://doi.org/10.1016/j.vetimm.2012.05.013
Sergeant E.S.G., McAloon C.G., Tratalos J.A., Citer L.R., Graham D.A., More S.J. Evaluation of national surveillance methods for detection of Irish dairy herds infected with Mycobacterium avium subspecies paratuberculosis. Journal of Dairy Science 2019; 102: 1–14. https://doi.org/10.3168/jds.2018-15696
Varges R., Marassi C.D., Oelemann W., Lilenbaum W. Interference of intradermal tuberculin tests on the serodiagnosis of paratuberculosis in cattle. Research in Veterinary Science 2009; 86 (3): 371-372. https://doi.org/10.1016/j.rvsc.2008.08.006
1. An animal has an ELISA test result of positive and a subsequent faecal PCR result of negative. The herd is not known to be infected. What is your Johne’s risk assessment of the animal?
A. Test-positive
B. Suspect
C. Test-negative
D. Insufficient information
2. A herd has completed one whole herd test with all ELISA results of negative. How would you advise the owner to approach controlling the risk of Johne’s disease?
A. The herd is free of infection, so no need to control spread of infection
B. Infection is unlikely, so concentrate on bio-exclusion (keeping infection out of the herd)
C. Confidence in just one negative herd test result is low, so concentrate on bio-containment (reducing spread within the herd)
D. Balance of bio-exclusion and biocontainment measures, according to owner’s priorities
3. A large herd in the IJCP has tested all except five animals for JD, and now, on December 1, has had a TB test. How should the five animals be tested?
A. Tested by PCR
B. Tested by ELISA, with any positive animals re-tested by ELISA after 90 days
C. Tested by ELISA, with any positive animals tested by PCR
D. Cull without testing
4. How should animals being brought into a herd be tested?
A. ELISA test before entry
B. Hold in isolation after entry until tested by ELISA
C. Assess the test history of the herd where the animal was born
D. Do not test this animal in case it is infected
Answers: 1B; 2D; 3B; 4C.
















