Large animal - July 2020
Equine faecal egg counts _ their role in reducing anthelmintic resistance
Cara Heatley BSc, a veterinary nurse at Mourne Veterinary Clinic, Kilkeel, Co. Down provides a comprehensive account of the role of faecal egg counts in reducing anthelmintic resistance in horses
Faecal worm egg counts (FWECs) are a vital tool to help reduce anthelmintic resistance in horses. They can identify which horses require anthelmintic treatment in order to reduce the number of infective larvae present in pasture. Additionally, they can distinguish between strongyle and ascarid presence in faecal samples. FWECs do, however, have restrictions in their use. They are unable to identify pre-patent stages of worms that are pathogenic or to determine individual horse disease risk. Also, there is a weak correlation between egg count and worm burden. Despite these limitations, FWECs are a vital diagnostic tool. Used in combination with suitable pasture management techniques, they are an essential means of helping to reduce anthelmintic resistance.
Deworming horses is a standard form of husbandry that all owners will be familiar with. The three anthelmintic drug classes used to treat horses are: benzimidazoles (eg. fenbendazole); tetrahydropyrimidine pyrantel; and macrocyclic lactones (ivermectin and moxidectin) (Peregrine et al, 2014). However, recommended dosing guidelines have changed quite dramatically in recent years. In the past, all horses were treated at regular intervals without knowing the extent of how many worms they hosted at any given time. This practice has resulted in certain endoparasites becoming resistant to several anthelmintic drugs (Dowling, 2018). Recommendations for parasite control has now been re-examined in order to incorporate differing parasite focus. Previously, Strongylus vulgaris was the most pathogenic endoparasite to horses. As S. vulgaris eggs take two months to reappear in the environment, horse owners were advised to treat their horses every two months. This approach prevented S. vulgaris eggs from being shed in pastures and, therefore, was very effective at preventing S. vulgaris infection in horses (Neilsen et al, 2019). Presently, S. vulgaris infection is very uncommon in managed horse populations.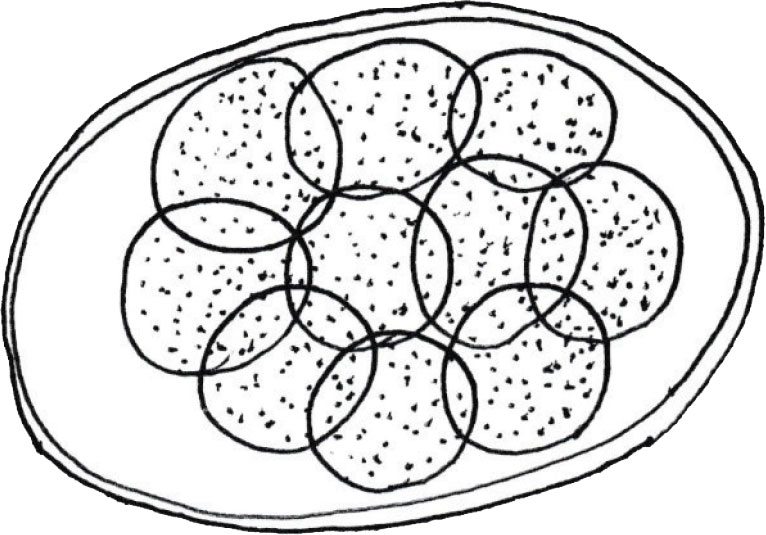
Figure 1: Typical strongyle egg (author’s illustration).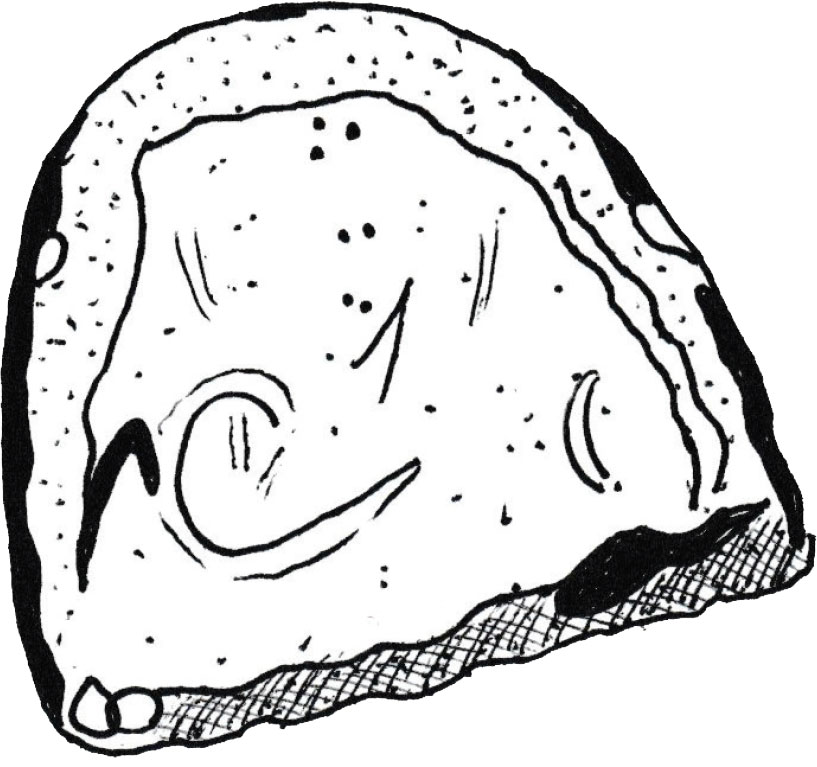
Figure 2: Parascaris equorum egg (author’s illustration).
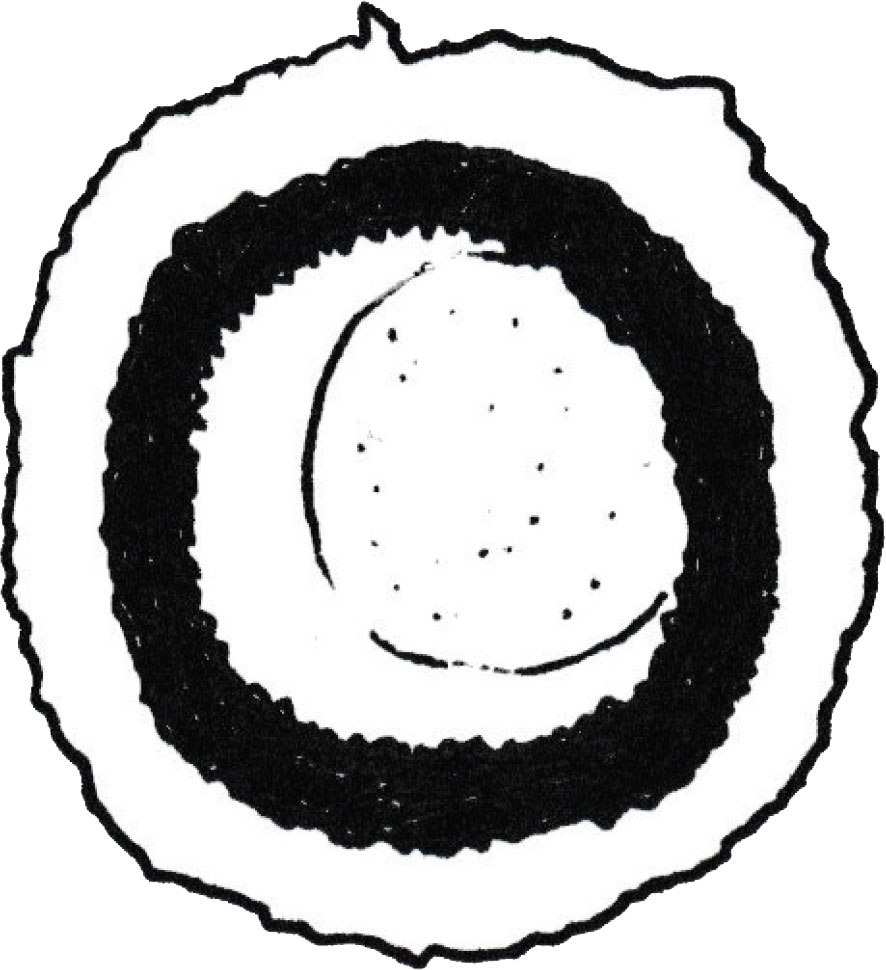
Figure 3: Anoplocephala perfoliate egg (author’s illustration).
The current primary equine parasitic pathogens are cyathostomins (small stronglyes). Other notable pathogenic parasites are Parascaris equorum (mainly in foals and weanlings) and Anoplocephala perfoliate, which is a common cause of ileal colic in horses (Neilsen et al, 2019). Each of these parasites have lifecycles quite different to S. vulgaris, therefore the dosing strategy of every two months would not be suitable. Cyathostomins have a pre-patent period of two to three months; that of P. equorum is 2.5 to three months; whereas A. perfoliata is two months (Foreyt, 2001). Unfortunately, many years of routinely deworming horses has resulted in high levels of anthelmintic drug resistance in cyathostomin and P. equorum populations (Peregrine et al, 2014). This is an indication that deworming is not an effective standalone strategy and that other tactics must be implemented. Cyathostomins are globally resistant to benzimidazoles and to a lesser extent macrocyclic lactones. P. equorum has remained susceptible to all three anthelmintic drug classes. There has not been any reported resistance to pyrantel in the UK or Ireland, however it has been described in the US (Coles, 2009).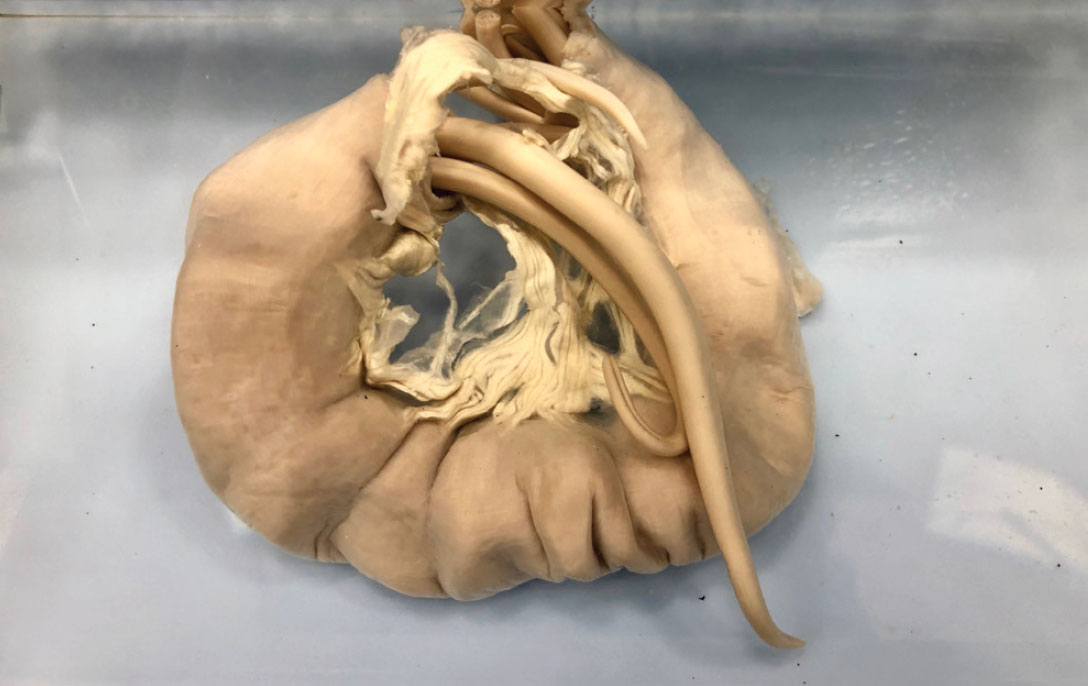
Figure 4: P. equorum in a yearling pony’s small intestine. Photo: Karen Dunne.
RESISTANCE
Sangster (1999) defines resistance as the ability of worms in a population to survive drug treatments, that are generally effective against the same species and stage of infection at the same dose rate. Resistance to equine deworming products is of growing concern. One reason for this is that horses are often being dewormed when they might not necessarily need treatment. Many horse owners are unaware of the implications of resistance, or perhaps are unsure of other options available (Hobson, 2013). Horse owners have become reliant on anthelmintics and parasiticides, while overlooking other valuable strategies that can help control parasites. Nematodes (roundworms) and trematodes (flukes) are becoming increasingly resistant to several types of anthelmintics. Cyathostome nematodes have become particularly resistant to benzimidazoles which means that this class of anthelmintics is no longer indicated for equine use (Merial Equine Health, 2011).
When a horse receives anthelmintic treatment, susceptible worms are eliminated from the horse. Worms that have not been eliminated, and therefore are not susceptible to that treatment, possess genes for resistance to the anthelmintic drug given to the horse. The consequence of this is that these worms will produce resistant offspring. This is referred to as a resistant strain.
The reasons for genetic resistance are unclear. Possibilities for resistance could be: the result of a mutation; or resistant genes may be present at a low frequency before treatment has been provided. Resistant worms are regarded as having a greater ability to prevent uptake of the anthelmintic drug (which is rapidly metabolised and excreted). It is also a possibility that they are able to evade the action of the drug by changes in receptor sites or by transferring to different biochemical pathways (Merial Equine Health, 2011). Research into the genetics of anthelmintic resistance has progressed little over the years. The more effectively helminths are controlled with anthelmintic treatment, the likelihood of resistance increases. With every treatment, resistant worms are left behind. When these worms reproduce, the offspring will possess resistant genes (Sangster, 1999).
FAECAL WORM EGG COUNTS
FWECs are endorsed as an effective deworming strategy with the aim of prolonging the efficacy of anthelmintics while also reducing the costs of parasite control (Rose Vineer et al, 2017). As drug resistance becomes more prevalent, FWECs are being used to determine which adult horses will require anthelmintic treatment. This is commonly referred to as selective therapy (Hallowell-Evans, 2016). During optimal environmental conditions for the development of infective strongyles larvae (usually spring and summer), FWECs are used to identify which horses exceed a predetermined cutoff value (see Table 1). Horses below the cut off value are either not treated or treated less often. The purpose of this idea is to reduce the overall number of horses that receive anthelmintics treatment. Doing so reduces selection pressure for anthelmintic resistance by providing refugia. This is the proportion of the total parasite population that is not selected for treatment (Food and Drug Administration, 2019).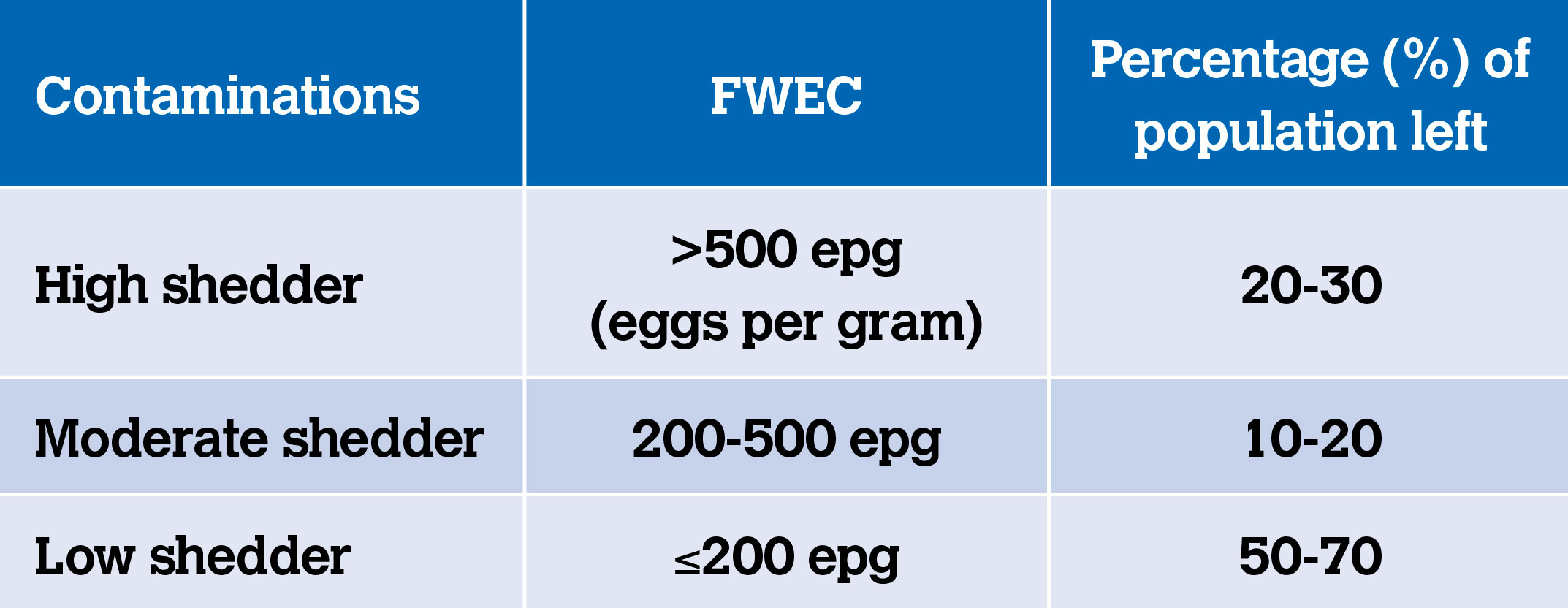
Table 1: Categorising horses according to shedding potential (Neilsen et al, 2019).
Figure 5: Faecal flotation. Photo: Karen Dunne.
FWECs are performed using a small faecal sample (5-10g). They are used during routine monitoring of parasite status and also for identifying infected horses for targeted treatment and investigation of a newly purchased horse (Snalune, 2018). A FWEC test is inexpensive and straightforward to implement and is often performed in-house in equine practices. It usually requires 5-10 minutes to complete (Lane, 2015). The test should be carried out both on day zero (before anthelmintic treatment) and day 14 (post-treatment).
In equine practice, the McMaster counting technique is commonly used for identifying high-egg shedders. This method enables the operator to demonstrate and count helminth eggs (Royal Veterinary College, 2019n). A counting chamber is filled with faecal suspension which is then assessed using a microscope. Detailed instructions advising how to perform the McMaster counting technique are available in Table 2. The theory behind this method is that, if a known weight of faeces and volume of flotation fluid are used to create the suspension, the number of eggs per gram (epg) can be calculated. The engraved compartments in a McMaster slide ensure that it is easy for operators to know where to count eggs ie. within the chambers (Royal Veterinary College, 2019f). Analysing FWEC results requires expertise. The operator will be required to identify different types of eggs (see Figures 1-3) and will also have to be aware of various factors that can affect results. It is not possible to distinguish between cyathostomins and S. vulgaris via FWEC due to their eggs possessing an extremely similar appearance. Instead, the infective larvae can be identified following faecal culture (Klei, 2019).
• Faecal egg count reduction test
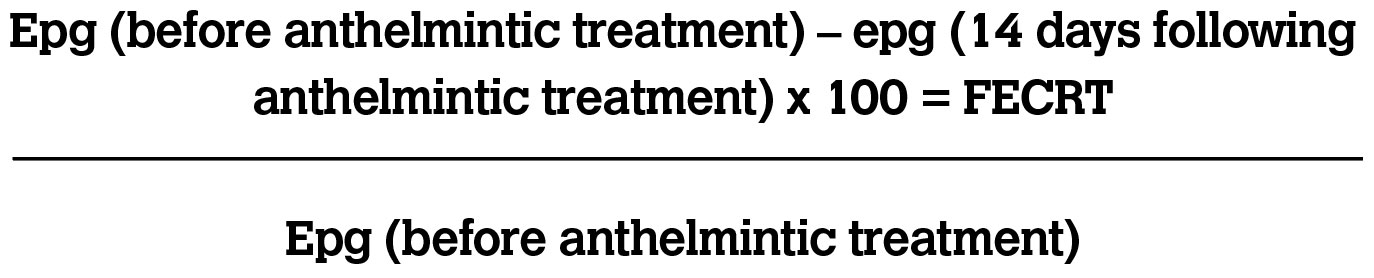
The faecal egg count reduction test (FECRT) is considered the gold standard for defining the anthelmintic susceptibility of P. equorum and cyathostomin infections (Peregrine, 2014). Before providing an anthelmintic treatment to a horse, a faecal sample should be collected to perform a FECRT. The deworming treatment is then administered, with a second faecal sample being collected 14 days following this. The number of eggs found in both samples is used to determine the reduction in FWEC. It is recommended that a FECRT is performed on at least six horses on each farm. A calculation (see Table 2) is used to assess the percentage reduction in FWEC for each horse. The mean reduction is then determined using the figure for each horse which will provide the percent reduction for the farm. The most suitable method for performing a FECRT is the modified Wisconsin technique. It is similar to the McMaster technique; however, it involves centrifugation and does not require specialised slides (Neilsen et al, 2019).
Dowling (2018) explains that it is meaningless to test faeces whilst previous anthelmintic treatments are still active. Each wormer will last for different time periods (see Table 3). This means that depending on the active ingredient(s) present in the wormer given, faeces should not be tested until the drug is no longer present in the horse.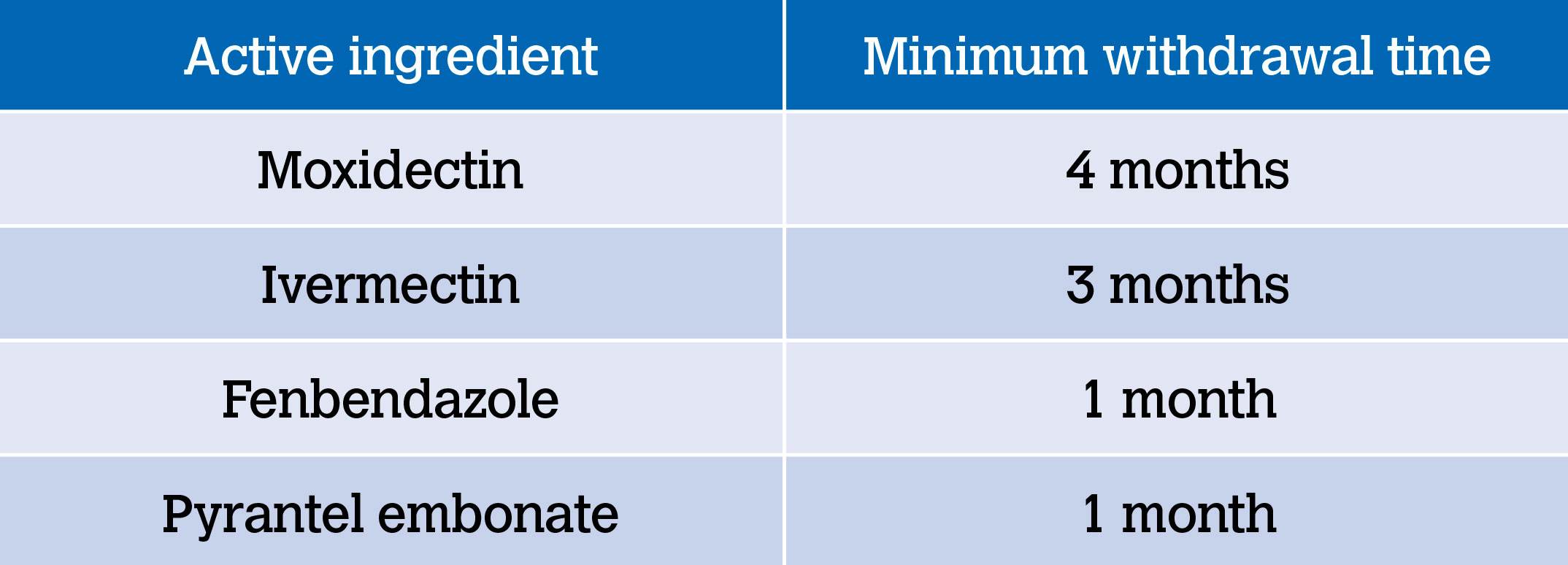
Table 3: Anthelmintic treatment efficacy times (Neilsen et al, 2019).
McMaster Technique (adapted from Royal Veterinary College, 2019)
-
Step 1: Place four grams of fresh faeces into a beaker or a plastic container.
-
Step 2: Add 56ml of flotation fluid to the container. The following solutions can be used as a flotation fluid for general purpose: saturated salt solution; salt/sugar solution; or sodium nitrate.
-
Step 3: Stir the entire contents using a spatula, fork or tongue depressor.
-
Step 4: Pour the newly formed faecal suspension through a tea strainer or a double layer of cheesecloth into an empty container.
-
Step 5: Using a Pasteur pipette, stir the filtrate in the second container. Then using the same pipette, remove a small amount of the contents whilst the filtrate is being stirred.
-
Step 6: Stir filtrate and fill the first chamber with the sub sample, repeating this step for the second compartment.
-
Step 7: Allow the sample to stand for five minutes to give the eggs time to float to the surface. Debris will also descend to the bottom of the chamber. If eggs are counted after this time period, the result may be inaccurate as the flotation fluid may distort or destroy eggs.
Examination
Using the 10x10 magnification on a microscope, identify and count all visible eggs within the engraved areas of both chambers.
Calculation
Multiply the total number of eggs by 50. This will result in the eggs per gram (epg) of faeces.
Example calculation:
Chamber 1: 4 eggs
Chamber 2: 5 eggs
Chamber 1 + Chamber 2 = 9 eggs
9 eggs x 50 = 450 epg
Alternatively, the eggs in one chamber only can be counted. This figure should then be multiplied by 100.
Example calculation:
Chamber 1: 5 eggs
5 eggs x 100 = 500 epg
• Automatic FWECs
In these technology-focused times, FWECs are not the only method that can be used to count worm eggs. Smartphone-based egg counting applications are a novel way to perform FWECs. Most are currently only based in the USA and are only beginning to be rolled out to veterinary professionals. One example is Parasight™, which is an FWEC method that identifies and counts strongyles and ascarid eggs in under five minutes. Parasight™ (2019), the company, states that its technology operates with less variability and more accuracy than traditional McMaster methods. It uses an automated algorithm and software imaging technology to provide eggs per gram results and fluorescent images of present parasites. Parasight™ has introduced a system for horse-owners to operate. Saeed and Jabbar (2017) explain that modern smartphones contain powerful in-built sensors which enable them to be used as an alternative to professional diagnostic laboratory equipment. However, the use of smartphones in the diagnosis of parasitic burdens remains in its infancy.

Figure 6: Horses grazing. Photo: Karen Dunne.
OTHER STRATEGIES
No single discipline has conquered worms, which means that a multi-disciplinary approach is vital (Sangster, 1999). In 1999, legislation in Denmark was amended to render anthelmintic drugs available by prescription only (Loving, 2014). Their use for routine, prophylactic treatment was prohibited. The purpose of this strategy was to encourage horse owners to consult with their veterinary practitioners before they were able to treat their horses. A 2004 survey that targeted Danish equine veterinary practices, was conducted to determine whether this strategy was effective in reducing anthelmintic resistance in horses. Ninety seven per cent of respondents stated that they used FWECs to diagnose and investigate larval cultures (Neilsen et al, 2005). Results of the survey suggested that limiting equine anthelmintics to prescription-only status actually increased the level of strongyle surveillance. Hobson (2013) states that the most important part of anthelmintic control is the application of farm management techniques. Examples of these techniques include: daily removal of faeces; cross grazing with sheep or cattle; composting manure; rotating paddocks; and avoiding high stocking densities.
• Pasture management
University of Liverpool (2019) states that the principle of parasite control is to prevent pasture contamination. The purpose of this is to prevent parasites from fulfilling their lifecycle in the host. The British Horse Society (2017) explains that horses will spend up to 16 hours a day grazing which means that it is evident that they will encounter infective larvae as they graze. In order to help decrease the occurrence of this happening, it is recommended that droppings are removed from the pasture twice-weekly. While this is labour-intensive for those performing the task, it has been proven to be an effective method of reducing FWEC numbers in grazing horses (University of Liverpool, 2019).
• Composting manure
Davis et al (2019) state that composting manure can help control the risk of parasite exposure. In temperatures over 40oC for a minimum of one week, strongyle larvae in manure are destroyed. Large-scale compost systems can reach temperatures of up to 60-70oC within three to five days (Trautmann, 1996).
• New arrival checks
The American Association of Equine Practitioners (Nielsen et al, 2019) recommends that a FWEC be performed on new arrivals to farms or stables. The most commonly used anthelmintic treatment should be administered; 14 days following this, a second FWEC should be completed in order to determine the FECRT. Ideally, this should be carried out in confinement until it is confirmed that the new horse is not a high shedder. An additional benefit of this test is that it will determine the efficacy of the wormer used.
• Rotating paddocks
The rotation of pasture provides adequate time for infective eggs and larvae to die on unused pasture. University of Liverpool (2019) recommends that at least three months is given for each rest period.
• Stocking densities
The British Horse Society (2017) advises to stock pastures with a maximum of two horses per hectare on permanent grazing areas. Overstocked pastures can result in a high concentration of droppings. This leads to an increased potential of horses ingesting worm larvae.
• Mixed species grazing
Introducing other species of animals into the pasture that horses normally graze on can help control parasite numbers. Whilst grazing, cattle and sheep will ingest eggs and larvae that are unable to survive in hosts other than the horse (University of Liverpool, 2019). Barker (2015) suggests that donkeys are not grazed on the same pasture as horses. The lungworm Dictyocaulus arnfieldi is non-pathogenic to donkeys but can result in lung damage in horses.
CONCLUSION
Equine veterinary practitioners are being challenged with a lack of anthelmintic options. Increasing occurrences of multi-drug resistance has resulted in VPs and horse-owners looking elsewhere for parasite control (Peregrine et al, 2014). FWECs are a cost-effective diagnostic tool that can identify low, moderate and high worm-egg shedding horses. Additionally, they can also be used for FECRTs which can assess resistance to specific anthelmintic treatments. Increasingly, veterinary practices are offering FWECs to their clients before prescribing deworming drugs. Not only are they inexpensive for clients, they are also quick and relatively simple to perform for veterinary staff. Equipment required to perform the McMaster test is easily sourced and will normally be present in an equine hospital. From researching different automated FWEC smartphone apps suggests it is possible that the FWEC market may branch out to targeting clients themselves in the future.
While FWECs are a key element in regards to reducing anthelmintic resistance in horses, they will need to be used in combination with other tactics. Equine strongyle parasites begin their lifecycles in manure piles, which means that this is an area that needs to be targeted (Neilsen et al, 2019). There are various methods that can be used to help reduce worm egg contamination in pastures. They require manual labour and can be weather reliant; therefore their efficacy will depend on individual circumstances. Reinemeyer (2009) explains that research has confirmed that eradication of equine parasites is impossible. The focus instead should be on implementing management procedures that reduce transmission. In regard to anthelmintic treatments, only those who have demonstrated moderate to high shedding numbers should be treated. The timing of dosing should also be much less frequent in comparison to traditional dosing advice in an effort to combat drug resistance.
Acknowledgements
The author extends thanks to Karen Dunne for permitting the inclusion of her photographs in this article.
- Barker K. 2015. Equine pasture management [online]. Available from: https://www.vettimes.co.uk/app/uploads/wp-post-to-pdf-enhanced-cache/1/equine-pasture-management.pdf [accessed 15 April 2019].
- British Horse Society. 2017. Advice on pasture management [online]. Available from: www.bhs.org.uk/advice-and-information/horse-health-and-sickness/pasture-management [accessed 15 April 2019].
- Coles G. 2009. Anthelmintic resistance in equine worms [online]. Available from: https://www.vettimes.co.uk/article/anthelmintic-resistance-in-equine-worms/ [accessed 15 April 2019].
- Davis JG, Swinker AM and Smith C. 2019. Horse manure management [online]. Available from: https://lpelc.org/horse-manure-management/ [accessed 15 April 2019].
- Dowling D. 2018. It’s all about the poo: Selective worming for adult horses [online]. Available from: https://www.theirishfield.ie/horse-sense-it-s-all-about-the-poo-selective-worming-for-adult-horses-423893/ [accessed 29 January 2019].
- Drudge JH and Lyons ET. 1966. Control of internal parasites of the horse. Journal of the American Veterinary Medical Association, 148(4), pp.378-83.
- Food and Drug Administration. 2019. Antiparasitic resistance in cattle and small ruminants in the United States: how to detect it and what to do about it [online]. Available from: https://www.fda.gov/downloads/animalveterinary/resourcesforyou/ucm347442.pdf [accessed 19 March 2019].
- Foreyt WJ. 2001. Parasites of horses. In: Foreyt, W.J., ed. Veterinary parasitology: reference manual. 5th ed. Iowa: Blackwell, pp. 121 – 136.
- Gould JC, Rossano MG, Lawrence LM, Burk SV et al. 2012. The effects of windrow composting on the viability of Parascaris equorum eggs. Veterinary Parasitology, 191(1-2), pp. 73-80.
- Hallowell-Evans C. 2016. What do worm egg counts tell you? In: BEVA Congress 7-10th September 2016, ICC Birmingham [online], pp. 53-59. Available from: https://www.ivis.org/proceedings/beva/2016/BEVA2016.pdf [accessed 21 January 2019].
- Hobson L. 2013. Developing a strategic worming programme. Veterinary Ireland Journal, 3(7), pp. 386-388
- Klei TR. 2019. Large strongyles in horses [online]. Available from: https://www.msdvetmanual.com/horse-owners/digestive-disorders-of-horses/gastrointestinal-parasites-of-horses#v3218205 [accessed 14 April 2019].
- Lane C. 2015. The veterinary nurse’s role in implementing targeted strategic worming for horses. Veterinary Nursing Journal, 30(4), pp. 111-115.
- Loving NS. 2014. Prescription-only parasite control in Denmark [online]. Available from: https://equimanagement.com/news/prescriptiononly-parasite-control-denmark-21462 [accessed 6 March 2019].
- Merial Equine Health. 2011. Equine parasites and their control. Berkshire: Merial.
- Nielsen MK, Monrad J and Olsen SN. 2005. Prescription-only anthelmintics – a questionnaire survey of strategies for surveillance and control of equine strongyles in Denmark. Veterinary Parasitology, 135(2006), pp. 47-55.
- Neilsen MK, Mittel L, Grice A, Erskine M et al. 2019. AAEP parasite control guidelines [online]. Available from: https://aaep.org/sites/default/files/Guidelines/AAEPParasiteControlGuidelines_0.pdf [accessed 29 January 2019].
- Parasight. 2019. FAQs for veterinary professionals [online]. Available from: https://poop2proof.com/pages/faqs-for-veterinary-professionals [accessed 14 March 2019].
- Peregrine AS, Molento MB, Kaplan RM and Nielsen MK. 2014. Anthelmintic resistance in important parasites of horses: does it really matter? Veterinary Parasitology, 201(1-2), pp.1-8.
- Rose Vineer H, Vande Velde F, Bull K, Claerebout E et al. 2017. Attitudes towards worm egg counts and targeted selective treatment against equine cyathostomins. Preventative veterinary medicine, 144(2017), pp. 66-74.
- Royal Veterinary College. 2019. McMaster egg counting technique [online]. Available from: https://www.rvc.ac.uk/review/parasitology/eggcount/Exam1.htm [accessed 7 April 2019].
- Saeed MA and Jabbar M. 2017. ‘Smart diagnosis’ of parasites using smartphones. Journal of Clinical Microbiology [online], 10(12). Available from: https://jcm.asm.org/content/jcm/early/2017/10/12/JCM.01469-17.full.pdf [accessed 14th April 2019].
- Sangster NC. 1999. Anthelmintic resistance: past, present and future. International Journal for Parasitology, 29(1), pp.115-124.
- Snalune K. 2008. Equine internal parasites: Their types and management [online]. Available from: https://www.vettimes.co.uk/app/uploads/wp-post-to-pdf-enhanced-cache/1/equine-internal-parasites-their-types-and-management.pdf [accessed 22 January 2019].
- Trautmann N. 1996. Compost physics [online]. Available from: http://compost.css.cornell.edu/physics.html [accessed 15 April 2019].
1. The three anthelmintic drug classes used to treat horses are:
a. Benzimidazoles, tetrahydropyrimidine pyrantel and macrocyclic lactones
B. Benzimidazoles, probenzimidazoles and salicylanilides
C. Macrocyclic lactones, amino-acetonitrile derivatives and tetrahydropyrimidine pyrantel
D. Tetrahydropyrimidine pyrantel, macrocyclic lactones and probenzimidazoles
2. A high shedder will produce a FWEC result of:
a. >500 E.P.G
B. >300 E.P.G
C. >400 E.P.G
D. >200 E.P.G
3. Droppings should be removed from pasture:
a. Weekly
B. Daily
C. Bi-weekly
D. Monthly
4. Over a minimum of one week, strongyle larvae in manure are destroyed in temperatures over:
a. 60-70oC
B. 50oC
C. 40oC
D. 80oC
5. On permanent grazing areas, it is advisable to stock pastures with a maximum of:
a. 2 horses per hectare
B. 3 horses per hectare
C. 4 horses per hectare
D. 6 horses per hectare
















