Best practice protocols for use of antiparasitic drugs on farms in Ireland
Dr J. G. Beechinor MVB, MVM, MSc, PhD, CDipAF, MRCVS, director of veterinary sciences, Health Products Regulatory Authority, offers some tips on developing best practice protocols for the use of antiparasitic drugs on farms in Ireland
From January 28, 2022, it will be necessary for farmers to obtain a veterinary prescription before purchasing antiparasitic veterinary medicines for use in food-producing animals. This follows a review by a HPRA expert group in December 2019, which concluded that the medicines concerned should be changed to ‘supply under veterinary prescription’ to comply with the legal criteria in Regulation 2019/6. The HPRA report found that:
- there is widespread resistance to anthelmintics in parasites of livestock in Ireland;
- anthelmintic resistance in parasites of other food-producing species has been demonstrated in European countries which have similar farming and animal husbandry conditions to those in Ireland;
- resistance in ectoparasites to several veterinary drug classes has been identified in European countries that have similar farming and animal husbandry conditions to those in Ireland; and,
- resistance to anti-coccidial veterinary medicinal products has been shown in European countries that have similar farming and animal husbandry conditions to those in Ireland.
This change brings Ireland into line with the rest of the EU on the supply of antiparasitic medicines for food-producing animals. Given the impact of the development on stakeholders, the logistics required to change the labelling of the affected medicines and the need to communicate developments to farmers, the change has been coordinated to coincide with other changes that come into effect with the application of Regulation 2019/6 in January 2022. The HPRA previously flagged this development to veterinarians, health care professionals, licensed merchants and farmers at the time of the original report, and again at intervals subsequently, including the placing of notices in the farming press in July 2021.
In June 2020, the Department of Agriculture, Food and the Marine established an Antiparasitic Resistance Stakeholder Group. One of their tasks is to “protect the efficacy of antiparasitics”, being consistent with a recommendation of the HPRA Report that a “multi-actor stakeholder approach be taken to elaborate national guidelines for sustainable parasite control, including the development of consistent scientifically-based advice on targeted selective treatments”.
With the imminent approach of the 2022 deadline, this article is intended to raise awareness among veterinarians on the principles involved in developing best practices for use of antiparasitics at individual farm level. Antiparasitic resistance is known to develop insidiously and the cumulative effect on animal growth in the herd or flock might not be apparent to farmers for several years, perhaps until identified as treatment failures. By this time, resistance in the target parasites is well-established and not easily rectified.
Few new classes of anthelmintics have been developed in recent years, and it is necessary to preserve the efficacy of existing classes. Therefore, akin to the (unrelated) challenge of addressing antimicrobial resistance, the objective must be to use available antiparasitic medicines only when they are needed and likely to be effective; to ensure that they are administered correctly; and to support their efficacy for the longer term by employing appropriate farm management/husbandry practices.
The HPRA understands discussions on the precise operation of national prescription controls are being considered currently by both DAFM and the Veterinary Council. This article does not address that aspect, but is focused on the scientific principles underlying how veterinarians may approach the development of treatment protocols for their farming clients.
Changes to product information
Many product labels for food-producing animals currently carry a table that indicates the dose rate of the medicine for particular live weight bands of the animals concerned. These dose rates are an easily accessible source of dose-rate information for farmers that can help reduce miscalculation of the dose rates and the risk of under-dosing.
However, in accordance with Regulation 2019/6, which applies on January 28, 2022, the information to be included on the packaging and labelling of new veterinary medicines will be restricted compared to what is currently permitted. Information on indications and dosing instructions will no longer be permitted and such information is expected to be moved from the immediate and outer product labelling to the package leaflet.
This measure is intended to simplify the extent of textual information that is required on the labels of medicines, and will, over time (based on new secondary legislation that is yet to be agreed), facilitate the provision of multi-lingual labelling and the use of standardised pictograms and abbreviations throughout the EU. This development will extend to include already authorised veterinary medicines within five years and is aimed at facilitating medicine availability by use of common labelling in Member States. This highlights the importance of carefully reading the package leaflet before prescribing veterinary medicines given the reduced information likely to be present on the product packaging.
Considerations in developing best practice guidance to control resistance
There are several reference sources available to help inform the prescription for a cohort of animals, including a number of recent review articles from Ireland, the UK and the European Medicines Agency (EMA):
- anthelmintic resistance in cattle stomach and gutworms: PC-Anthelmintic-Resistance-in-Cattle-Roundworms-2021-2.pdf (animalhealthireland.ie);
- cattle gutworms – the facts: PC-Cattle-Gutworms-2021.pdf (animalhealthireland.ie);
- lungworm: AHI-Bulletin-June-2021-FINAL.pdf (animalhealthireland.ie);
- roundworms in sheep: AHI-Parasite-Control-Sheep-Roundworms-2021.pdf (animalhealthireland.ie);
- sheep scab: AHI-Parasite-Control-Sheep-Scab-2021.pdf (animalhealthireland.ie);
- bovine coccidiosis – the facts: PC-Coccidia-2021.pdf (animalhealthireland.ie);
- sustainable control of parasites (in sheep): https://www.scops.org.uk;
- control of worms sustainably (in cattle): https://cattleparasites.org.uk;
- EMA reflection paper on anthelmintic resistance: Reflection paper on anthelmintic resistance (europa.eu);
- EMA reflection paper on resistance in ectoparasites: Reflection paper on resistance in ectoparasites (europa.eu).
In addition, relevant peer reviewed literature should be consulted when drafting supportive advice to control resistance. Charlier et al (2017) advised that devising new control programmes for anthelmintics is a complex task as they would have to take “into account effects of multiple interactions on parasite populations [including gastrointestinal nematodes, lungworms, protozoa and trematodes], which might be conflicting and different for each worm species”. They also noted that infection pressure with gastrointestinal nematodes varies throughout the year as a function of weather and farm management, and that control programmes needed to be re-evaluated and adapted regularly to maintain their efficacy in the face of climate change and farm management. A principal goal of current thinking is to promote ‘refugia’, leaving the better animals in a cohort untreated, in order to promote the maintenance of drug-sensitive parasites in the environment in the face of drug exposure from treated animals. Hodgkinson et al (2019) advised that the success of best practice recommendations for gastro-intestinal nematodes of livestock that promote targeted selective treatment to preserve refugia depend upon many factors, including “potential fitness costs of harbouring resistant alleles in the population, the existing level of resistance in the population, the genetic diversity of the parasite population exposed to the drug, mechanisms and modes of inheritance of resistance alleles, and the efficacy and frequency of treatment with a particular drug”.
Indeed, the complexity of developing best practice may be best understood in Figure 1(below).
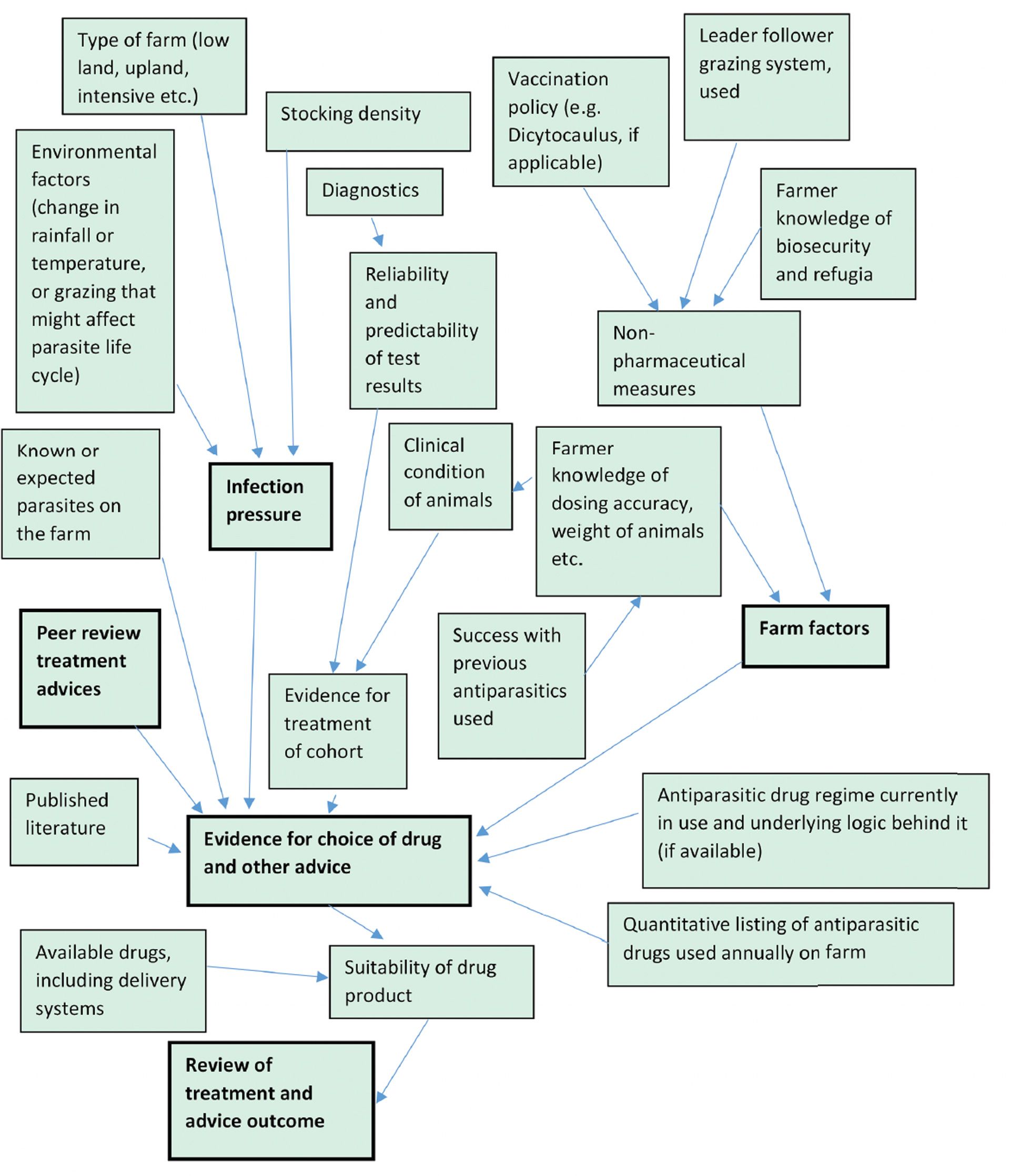
Figure 1. Considerations in developing protocols for use of antiparasitics on farm.
Before developing a treatment protocol, consideration should be given to:
- The farm or animal cohort for treatment, including likely parasite(s) present, resistance status (if known), recent climatic conditions in the locale, and infection pressure. This assessment should also consider the type of farm, grazing system use, stocking density, whether different species graze in the same farm etc.
- Diagnostic factors including the use, limitations and predictability of any laboratory findings (e.g., faecal egg counts, plasma pepsinogen levels). Were the available diagnostics taken from the cohort of animals being considered for treatment?
- Animal factors including clinical condition of individual animals, daily liveweight gain (if known).
- Farm management practices (e.g., is a leader/follower system used to reduce infection pressure? What efforts are being made to control exposure of young stock while promoting development of immunity to certain parasites? Are vaccines used to control hoose? Is quarantine of new stock applied?).
- Previous history of antiparasitic drug usage etc. is needed. This should include records of antiparasitic drugs used in the animal cohort or farm in recent previous years, including the totality of antiparasitic medicines used, as well as information on classes of drugs and in which cohorts they were used. Attention should be paid to whether drug combinations have been used, and/or whether an endectocide has been used to treat ectoparasites, as such use may have exerted unintended effects on resistance in nematodes.
- Details of the pharmaceutical forms of medicines used. Injectable ivermectins are used at a dose of 200mcg/kg bodyweight, while the dose of the substance topically is 500mcg/kg. On the other hand, convenience of administration might enhance user compliance with dosing instructions.
- Timing of treatments given and their likely impact on refugia, given the life cycle stage of the parasites concerned at time of treatment.
- Farmer knowledge of dosing accuracy, biosecurity measures and the concept of refugia are also important, in order to ensure that any medicine administered is used as intended, and that non-pharmaceutical control measures are fully exploited. In the case of elite health status herds, quarantine and treatment of new stock before joining the herd or flock should be considered.
It is unlikely that a single formulaic best practice protocol will be available to address individual farm needs throughout the country. As with developing other ‘best practices’, veterinarians are encouraged to research the scientific literature and readily available peer-reviewed advices. The approach to be taken for an individual farm should be tailored to that holding, and should take into consideration the latest research and advice, as well as the outcome of ongoing treatment protocols. Furthermore, local climatic factors and individual farming situations can change abruptly and must be considered and reviewed on a periodic basis.
Conclusion
Long-awaited regulatory initiatives to enhance the availability of veterinary medicines transnationally within the EU will result in certain instructions for use of veterinary medicines being re-located from product labelling to package leaflets, which might not always be readily available to users. While this development may take up to five years to be implemented for currently available medicines, it will apply to new medicines from January 28, 2022. Veterinarians will need to educate users to these changes, in order to ensure that the products are used properly.
Historically, many antiparasitic drugs have been self-selected by farmers in Ireland, but that regime is coming to a close. From January 28, 2022, veterinarians will have the unique responsibility for prescribing antiparasitics nationally. This development presents new opportunities to halt the development of antiparasitic resistance in food-producing animals. However, this is not an easy task. Certain parasitological maxims governing the use of anthelmintics that were considered best practice a few decades ago have been shown to be out-dated; the emphasis now is on evidence-based diagnosis, targeted selective treatment and refugia-based approaches to conserve susceptible alleles in target parasites. However, this approach to arrest the development of resistance requires careful consideration, stringent application and regular review. Even then, scientific knowledge gaps remain and success in halting the development of resistance is not guaranteed. A holistic approach that is informed by diagnostics, treatment and farm records, as well as farm management practice audits and taking into account latest best practices and peer-reviewed literature should assist in the development of tailored, evidence-based protocols to address individual farm needs. Whatever protocols are used, they will need periodic review and fine-tuning to ensure their continuing utility.
References
Charlier, J., Thamsborg, S. M., Bartley, D. J., Skuce, P. J., Kenyon, F., Geurden, T., Hoste, H., Williams, A. R., Sotiraki, S., Höglund, J., Chartier, C., Geldhof, P., van Dijk, J., Rinaldi, L., Morgan, E. R., von Samson‐Himmelstjerna, G., Vercruysse, J. and Claerebout, E. 2017. Mind the gaps in research on the control of gastrointestinal nematodes of farmed ruminants and pigs. Transboundary and Emerging Diseases, 65(S1); 217-234.
Hodgkinson, J.E., Kaplan, R.M., Kenyon, F., Morgan, E.R., Park, A. W., Paterson, S., Babayan, S.A., Beesley, N.J., Britton, C., Chaudhry, U., Doyle, S.R., Ezenwa, V. O., Fenton, A., Howell, S.B., Laing, R., Mable, B. K., Matthews, L., McIntyre, J., Milne, C.E., Morrison, T.A., Prentice, J.C., Sargison, N. D., Williams, D.J.L., Wolstenholme, A. J. and Devaney, E. 2019. Refugia and anthelmintic resistance: concepts and challenges. International Journal for Parasitology: Drugs and Drug Resistance, 10; 51-57.










