Large animal - January 2019
Antimicrobial drug use in calves – implications for antimicrobial resistance
Dr Bernadette Earley, Dr Anastasio Arguello, and Dr Mark McGee from Teagasc Grange Animal & Grassland Research and Innovation Centre, Co. Meath, and Aidan Murray, Teagasc, Letterkenny, Co. Donegal explore antimicrobial resistance implications on commercial beef and dairy farms in Ireland
Concern about the use of antimicrobials in food-producing animals is increasing. The following guidelines are recommended to maintain acceptable levels of antimicrobial usage on beef and dairy farms:
- Develop a herd-health plan in consultation with your veterinarian and Teagasc adviser;
- Pay attention to colostrum feeding, animal nutrition and animal purchasing policies;
- Vaccinate animals to reduce the need for antimicrobials and use alternatives to antimicrobials when available;
- Only give antimicrobials to animals under veterinary supervision;
- Do not use antimicrobials for growth promotion or to ‘prevent’ diseases in healthy animals; and
- Improve biosecurity on farms and prevent infections through improved hygiene and animal welfare.
What is antimicrobial resistance (AMR)?
Antimicrobial, derived from the Greek words anti (against), mikros (little) and bios (life), has a broader definition compared to just the term antibiotic and includes agents (both synthetic or natural), that act against bacteria, viruses, fungi and protozoa. In this paper, antimicrobial is taken to mean antibiotics (and their chemical derivatives) with an antibacterial range of action. Antimicrobial resistance (AMR) is the ability of bacteria (or microbes) to resist the effects of an antibiotic. AMR is one of the leading health concerns in human and veterinary medicine worldwide. AMR occurs when bacteria change in a way that reduces the effectiveness of drugs, chemicals, or other agents designed to cure or prevent infections. AMR can be intrinsic or acquired. Intrinsic or natural resistance is a trait of all bacteria belonging to a specific subspecies, species, genus, family or even higher taxonomic rank. Acquired resistance to antimicrobial drugs can develop in bacteria in two ways: genes can mutate, or genes from other bacteria can be horizontally transferred to them. AMR may cause treatment failure, both in humans and animals. This treatment failure results in a higher morbidity and mortality.
Monitoring antimicrobial usage
In Europe, various monitoring programmes have summarised antimicrobial consumption for animals through annual antimicrobial sales data (DANMAP, 2013; ANMV, 2014; MARAN, 2015). These programmes are structured to observe trends at the national level and for comparison of data between years and countries (ECDC/EFSA/EMA, 2015; EMA, 2015). However, a limiting factor of those programmes is that they are unable to provide more precise information, such as usage at farm level, variability between farms, etc.
Teagasc study on antimicrobial drug usage in calves
The main objective of the study described below was to quantify antimicrobial drug usage in calves using health treatment records from Irish suckler beef and dairy farms. In this study, antimicrobial usage refers to the exposure of a given animal or group of animals over a period of time to the active substance in each antimicrobial that was administered.
Data source
Data were obtained from a large-scale study on herd-level factors associated with the health and survival of calves on Irish farms (hereafter referred to as the herd-level study). Farmers, enrolled in the herd-level study, recorded birth, disease and health treatment, and death information on their calves using standardised recording sheets. Case definitions were provided to the farmers to assist with the classification of disease. Farmers completed and submitted the project recording sheets on a monthly basis. All health treatment data were reviewed. Long-acting antimicrobials administered more than seven days apart, or other medications administered more than three days apart, were classified as separate disease events. Crude morbidity was defined as calves being treated for at least one disease event, attributed to any cause, excluding injury. Calves treated for illnesses other than diarrhoea, pneumonia, navel infection, or joint infection/lameness were categorised as receiving treatment for ‘other’ disease events. The data collected were the antimicrobial trade name, the pharmaceutical form (oral solution, oral powder, parenteral solutions, tablets, bolus, etc.), the pack size (in L or ml for liquids, in g or kg for solids, in unit number for bolus or tablets, etc.), the total number of packages prescribed and dispensed to the farm, and the prescribed therapy (dose, administration frequency, duration).
Antimicrobial usage
Defined daily dose for animals (DDDvet) (mg/kg animal/day) and used daily dose (UDDvet) (mg/kg animal) were the technical units used to measure antimicrobial consumption. The DDDvet is defined as the average maintenance dose for the main indication in a specified species and it is provided by the by the European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) project for veterinary antimicrobial usage, whereas the UDDvet is calculated as the amount of an antimicrobial drug administered during a given period (days) divided by the number of calves at risk and their average live weight at the beginning of a treatment. In this way the UDDvet reflects the dose, truly administered by the producer. Treatment incidence (TI) was the indicator used to quantify antimicrobial usage. The TI provides a standardised technical unit of measurement that quantifies how many animals out of a theoretical group of 1,000 animals receive daily an antimicrobial treatment, and the calculations applied were:
TIUDD VET = Total active substance administered ×1000
UDDvet × standard BW × total calf days
TIDDD VET = Total Active Substance Administered ×1000
DDDvet × Standard BW × total calf days
The Population Correction Unit (PCU) is a measurement developed by the European Medicines Agency (EMA) and takes into account the animal population as well as the estimated weight of each particular animal at the time of treatment with antimicrobials. The milligrams (mg) of antimicrobial used per PCU was calculated.
Results
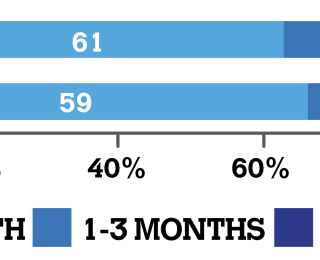
This study provides the first detailed information pertaining to on-farm usage of antimicrobials in suckler beef and artificially-reared dairy calves from birth to six months of age, in Ireland. A total of 123 farms (79 beef and 44 dairy), comprising 3,204 suckler beef calves and 5,358 dairy calves, representing 540,953 and 579,997 calf days at risk, respectively, were included in the study. All calves were raised on farm of origin and most of the studied herds were closed herds. In this study, only animals showing signs of disease were treated with antimicrobials and no mass administration of antibiotics was practiced. On beef farms overall, 12.7%, 5.7%, 2.9% and 20.4% of suckler beef calves were treated with antimicrobials for disease from birth to one month of age, one to three months of age, three to six months of age, and birth to six months of age, respectively. The corresponding values on dairy farms, overall, for calves treated with antimicrobials were 10.2%, 5.3%, 1.9% and 14.8%. The highest risk period for disease in the present study was between birth and one month of age with approximately two-thirds of all disease events occurring during this time period. This is reflected in the proportion of antimicrobials administered to calves at this time (Figure 1).
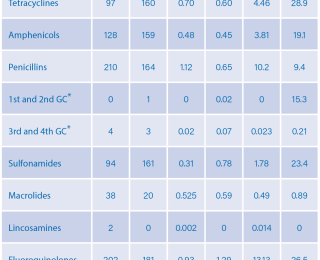
The classes of antimicrobials most frequently prescribed for beef and dairy calves were: tetracyclines, amphenicols, penicillins, 1st and 2nd generation cephalosporins (GC), 3rd and 4th GC, sulfonamides, macrolides, lincosamines, fluoroquinolone, aminoglycosides and spectinomycin (Table 1).
A total of 1,770 antimicrobial treatments were prescribed and administered to suckler beef (n=841) and dairy calves (n=929) between birth and six months. From birth to one month, the class of antimicrobial prescribed for most herds irrespective of type of farm, was penicillin (mostly amoxicillin) by the parenteral (non-oral) route (36.7% and 27.3%, beef and dairy, respectively). From one to three months of age, amphenicols (florfenicol) were the most prescribed class of antimicrobial for beef calves (17.7%) and tetracyclines (15.9%), mostly oxytetracycline, for dairy calves. Amphenicols (florfenicol) were prescribed more often in calves in the period from three to six months of age (11.4% and 16.0%, beef and dairy, respectively). The antimicrobials most prescribed for beef calves during the whole period (from birth to six months of age) were penicillins (mostly amoxicillin), tetracyclines (mostly oxytetracycline), amphenicols (florfenicol) and fluoroquinolones (enrofloxacin and marbofloxacin) (41.8%, 30.4%, 29.1% [13.9% and 25.2%] respectively). From birth to six months, penicillins (mostly amoxicillin), amphenicols (florfenicol), tetracyclines (mostly oxytetracycline) and fluoroquinolones (mostly enrofloxacin and marbofloxacin) were more frequently prescribed (34.1%, 29.6%, 22.7% [18.2% and 22.7%), respectively) for dairy calves. Due to their special surveillance in the context of AMR, the third and fourth generation cephalosporins were separated from other beta-lactams, and fluoroquinolones from other quinolones.
Fluoroquinolones were the most prescribed antimicrobials with 383 treatments, followed by penicillins (n=374), amphenicols (n=287) and tetracyclines (n=257). The third and fourth generation cephalosporins accounted for a total of seven treatments (Table 1). In the present study the mg/PCU was 8.03, 2.70, 1.43 and 7.25 for suckler beef calves for the treatment periods from zero to one, one to three, three to six, and from birth to six months, respectively. The corresponding values for dairy calves were 9.74, 3.72, 0.95, and 7.11 mg/PCU. The average cost of veterinary services was €41.25 and €43.37 per calf for beef and dairy calves, respectively; corresponding antimicrobial costs were €11.58 and €11.51 per calf.
Actions the farmer can take to keep antimicrobials working
- Only give antimicrobials to animals under veterinary supervision.
- Always give the right dose, and the number of treatments, as prescribed by your vet.
- Do not use antimicrobials for growth ‘promotion’ or disease ‘prevention’ in healthy animals.
- Do not use antimicrobials to treat viral disease.
- Do not use a ‘stronger’ antimicrobial as first-line treatment.
- Vaccinate animals to reduce the need for antimicrobials and use alternatives to antimicrobials when available.
- Improve biosecurity on farms and prevent infections through improved hygiene and animal welfare.
- In the case of medicines used in food-producing animals, ensure that the Animal Remedies Record is updated on each occasion that a veterinary medicine is administered.
Further information is available at www.agriculture.gov.ie/amr/
- List of Products containing DAFM Highest Priority Critically Important
- Antimicrobials (pdf 468Kb)
- Code of Good Practice Regarding the Responsible Prescribing & Use of ABs in Farm Animals (pdf 1,326Kb)
- Ireland's National Action Plan on Antimicrobial Resistance 2017-2020
- DAFM surveillance on AMR (doc 609Kb)
- European Commission Guidelines on the prudent use of antimicrobials in veterinary medicines
- European Commission – 5 year action plan
The World Health Organization (WHO) categorises antimicrobials used in human health as ‘critically important’, ‘highly important’ and ‘important’ to human health. The critically important antimicrobials (CIAs) are, therefore, the most important to human health. The CIAs are further categorised into Highest Priority CIAs (HP-CIAs) and High Priority CIAs. Given the importance of HP-CIAs in human health, these antimicrobials should NOT be used prophylactically or as first-line treatment in animals. They should only be used when there are no effective alternative antimicrobials available for the treatment of respective target species and indication.
Acknowledgements
Funding from the Department of Agriculture, Food and the Marine (Dr B Earley, project leader) under the Stimulus Fund (11/S/131) is gratefully acknowledged. The authors also wish to acknowledge the participating farmers, their Teagasc advisers, Cynthia Todd and Olivia Butler with data collection, and the administrative staff at Teagasc Grange for their support of this research.