UCD Research - January 2022
Test tube foals: A reality, but still a lot to learn
The new equine reproduction and fertility unit in UCD are now offering a clinical ovum pick up (OPU) service to Irish breeders while setting sights on important research questions to refine the process and ensure it is as physiological as possible
In association with


Niamh Lewis
BVM&S DACT DECAR PhD MRCVS is Assistant Professor in Equine Reproduction and Fertility in the UCD School of Veterinary Medicine and is supported by John Furlong BSc, an experienced human embryologist, technical officer and post-graduate researcher.
Niamh graduated from the School of Veterinary Medicine, University of Edinburgh in 2008 and spent several years in clinical practice focusing on equine reproduction in Ireland, Australia, UK, USA and Saudi Arabia. She then completed a residency programme at the University of Liverpool, subsequently becoming a European and American registered specialist in Equine Reproduction in 2015. Her PhD was focused on developing a metabolic approach towards optimising equine in vitro oocyte maturation and was a collaborative project between the University of Liverpool, University of Manchester and Texas A&M University. Niamh has established an Equine Assisted Reproduction Laboratory within the School of Veterinary Medicine to continue her research in equine assisted reproduction, specifically energy metabolism of the equine oocyte and embryo during in vitro maturation and culture and how it relates to developmental competence and health of resulting offspring.
Horses are one of the few species, besides humans, in which assisted reproductive technology (ART) has important clinical applications. In recent years, interest in the use of ARTs such as ovum pick-up (OPU) and intracytoplasmic sperm injection (ICSI) has rapidly increased worldwide with more than 3,000 procedures now being carried out annually in Europe alone. Furthermore, the horse can serve as a valuable model for the study of comparative reproductive biology as the mare has a similar oocyte ageing profile and follicular patterns to women. While there is established clinical success with the technology, there remains many unanswered questions that could improve the process and potentially maximise the health of the resulting offspring.
The clinical scenario
The use of OPU/ICSI was first driven by breeders wanting to produce offspring from sub-fertile mares that repeatedly failed to produce embryos from conventional embryo transfer (ET) and/or from stallions with low fertility. Due to increased efficiency of the technology breeders are now using it to maximise mare genetics and access sought after stallions with limited availability of frozen semen. While artificial insemination (AI) with a single straw can be successful in some cases, using a single straw for ICSI can result in multiple pregnancies. In fact, only a fraction of a straw is thawed for each ICSI session, and this fraction can be used for several mares.
The steps involved
1. Ovum Pick Up
OPU involves trans-vaginal aspiration of all visible follicles on the ovary. OPU can be performed throughout the year as long as the mare has a sufficient number of follicles present (ideally at least 15 that are 10mm or above). Each follicle is entered and flushed individually while stabilising the ovary per rectum. The expected recovery rate is in the region of 50-60 per cent. This procedure is well tolerated with minor complications such as rectal irritation, fever, mild colic and mild peritonitis reported in < one per cent of procedures and major complications and death occurring rarely (approx. ~1:1,000 cases). The retrieved immature oocytes are then shipped overnight to an ICSI laboratory (Figure 1). At present oocytes are shipped to Avantea in Italy but we hope that in late 2022 we may be able to offer our own ICSI services here at UCD.
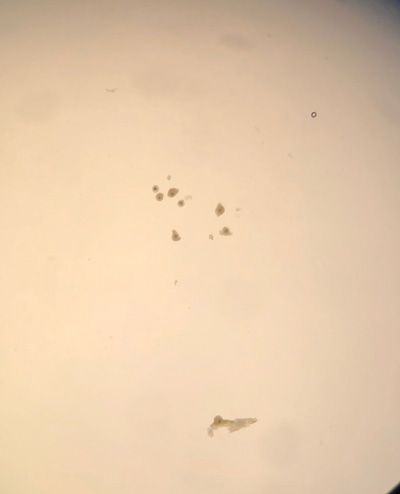
Figure 1: Oocytes obtained via OPU.
2. Maturation and ICSI
On arrival at the ICSI laboratory, the oocytes are placed in maturation media for approximately 26 to 30 hours to promote resumption of meiosis. The oocytes are then stripped of their cumulus cells and examined for the presence of a polar body which indicates they are at Metaphase II. Approximately 60 per cent of immature oocytes will reach Metaphase II and be suitable for sperm injection. A small section of a frozen semen straw from the chosen stallion is then thawed and semen is prepared for ICSI in order to select the most viable sperm cell. ICSI is then performed on each individual oocyte (Figure 2).
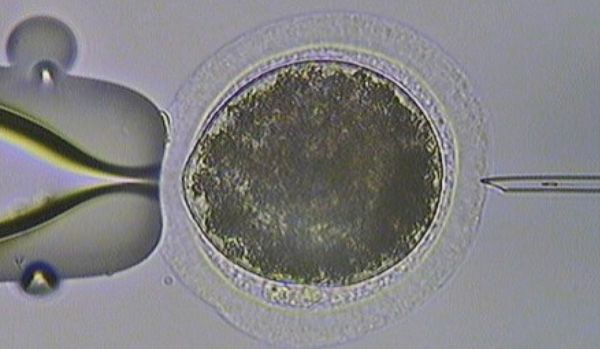
Figure 2: ICSI.
Embryo development and transfer
The injected oocytes are then allowed to develop in an incubator for six to nine days until they reach the blastocyst stage (Figure 3). The blastocyst rate per injected oocyte is approximately 20 per cent but can range from 0 per cent to 100 per cent. At this point, they are either frozen or transferred fresh to a recipient mare. If frozen, embryos are shipped to the location of the recipient mare where they can be thawed and transferred at a later date.
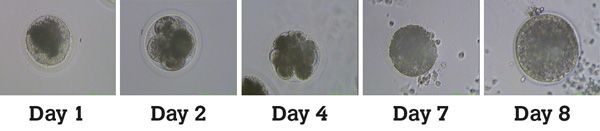
Figure 3: Embryo development after ICSI. The Day 8 image shows typical blastocyst features such as a visible trophectoderm layer and presence of a central cavity indicating a good quality embryo suitable for transfer or cryopreservation.
Expected results from OPU/ICSI
Approximately 75 per cent of OPU sessions will result in production of at least one embryo, with the average number of embryos produced being 1.7 to 2. There is a strong mare effect on the process; mares that yield a high number of embryos at the first attempt are likely to do so again, however the opposite is true for mares that fail to produce embryos. The biggest determinant of success is how many follicles the mare has and how many oocytes are retrieved but factors such as mare and stallion fertility, presence of uterine disease, fitness, and advanced age (>23 years) will influence results in individual cases.
Transfer of ICSI embryos results in similar pregnancy rates to those for fresh embryos flushed from a mare (approximately 70 per cent), however there is a slightly higher embryo loss rate so the actual live foal rate per embryo transferred is 50 per cent. While OPU/ICSI can be successful, it does come at an increased cost so the offspring produced should merit this expenditure.
Research direction and unanswered questions
Assisted reproduction technologies aim to mimic the periconceptual environment. As such, it is important to make sure they are optimised to the best of our knowledge. In the horse, there is a paucity of species-specific optimisation of the in vitro embryo production (IVEP) process. It is clear that good blastocyst rates and foal production can occur under the current conditions and anecdotal evidence exists of excellent performance in the resulting adult horses. This indicates that the equine oocyte and embryo is able to adapt to culture conditions. However, as is becoming clear in other species, this adaptation can occur at the detriment of long-term health outcomes. These are indices which have not yet been examined for foals produced by equine ART.
Particularly interesting to the research group at UCD is the fact that equine oocytes and embryos are currently cultured in media containing supraphysiological levels of glucose, levels that would ‘kill’ embryos of other species but in which equine oocytes and embryos seem to thrive. We know that equine oocytes are unique in this and also have particularly high levels of intracellular lipids. Across the domestic species and man, a ‘metabolic approach’ has proved useful in directing the optimisation of IVEP systems. Studying metabolism allows species-specific requirements to first be identified and then the culture system to be subsequently tailored to meet those needs. Dr Lewis has carried out novel work in this area characterising the energy metabolism pathways in use by cumulus oocyte complexes (COCs) during maturation and identifying the substrates used for ATP production1. Central to the metabolic approach is preserving mitochondrial function given their important role in fertilisation and maintaining normal embryo development. Dr Lewis has already demonstrated changes in mitochondrial function in the COC as a result of variations in glucose concentration during in vitro maturation2. Future work in UCD will explore what the functional importance of such changes may be and how to adjust medium composition to optimise mitochondrial function. The brand new ICSI facility in UCD will be well poised to produce embryos under varying conditions and utilise established methods such as transcriptomics and metabolomics to evaluate their developmental competence. It is also hoped that, pending sufficient funding, we can establish a research herd of mares so that we can carry out longitudinal studies on offspring health and performance and thus give further confidence to breeders who are utilising these technologies to produce champions of the future.
-
Lewis N, Hinrichs K, Leese, H.J. et al. Energy metabolism of the equine cumulus oocyte complex during in vitro maturation. Scientific Reports 2020; 10: 3493
-
Lewis N, Hinrichs K, Leese H.J. Glucose concentration during equine in vitro maturation alters mitochondrial function. Reproduction 2020; 160 227-237
In association with


Niamh Lewis
BVM&S DACT DECAR PhD MRCVS is Assistant Professor in Equine Reproduction and Fertility in the UCD School of Veterinary Medicine and is supported by John Furlong BSc, an experienced human embryologist, technical officer and post-graduate researcher.
Niamh graduated from the School of Veterinary Medicine, University of Edinburgh in 2008 and spent several years in clinical practice focusing on equine reproduction in Ireland, Australia, UK, USA and Saudi Arabia. She then completed a residency programme at the University of Liverpool, subsequently becoming a European and American registered specialist in Equine Reproduction in 2015. Her PhD was focused on developing a metabolic approach towards optimising equine in vitro oocyte maturation and was a collaborative project between the University of Liverpool, University of Manchester and Texas A&M University. Niamh has established an Equine Assisted Reproduction Laboratory within the School of Veterinary Medicine to continue her research in equine assisted reproduction, specifically energy metabolism of the equine oocyte and embryo during in vitro maturation and culture and how it relates to developmental competence and health of resulting offspring.









