UCD Research - May 2021
Basic alpaca husbandry and medicine
Victoria Rhodes, UCD Veterinary Hospital, University College Dublin; Fay Pooley, Scarsdale Vets, Derby, United Kingdom; and Marijke Beltman, UCD Veterinary Hospital, University College Dublin, outline some basic husbandry and medicine of alpacas
In association with

Victoria Rhodes
UCD Veterinary Hospital, University College Dublin
Fay Pooley
Scarsdale Vets, Derby, United Kingdom
Marijke Beltman
UCD Veterinary Hospital, University College Dublin
Keeping alpacas has become extremely popular across the world in recent years, with approximately 2,000 in Ireland. Alpacas are regarded as exotic animals under Irish legislation, meaning that drugs are not restricted like other food producing species.
The alpaca originates from South America in the dry, high altitude Andean Plains and has therefore had to adapt to the Irish climate and its wet, lush pastures. There are two types of alpaca kept in Ireland: Huacaya are the most popular and known for their fluffier fleece; Suri alpacas have a longer, silky fleece that parts down the spine. Alpacas are pseudo-ruminant with three compartments/stomachs aiding digestion of plant matter.
These compartments are referred to as C1, C2 and C3. C1 can be compared to the rumen in ruminants, with C2 most like the reticulum and C3 being partially like the abomasum with part of it containing gastric glands. There are commercial alpaca foods available and it is worthwhile recommending those to clients that have alpacas as these foods are tailored to the alpaca’s specific nutritional needs.
TB susceptibility
Alpacas are more susceptible to TB when compared to cattle and, therefore, appropriate PPE should be worn when dealing with sick alpacas. Alpacas with clinical TB can present in many ways with ill thrift being the most common. However, TB should be borne in mind for any presentation of illness. TB testing in alpacas can be done via blood testing.
Worming
Alpacas do not maintain a long lived immunity to common parasites like other farm species and are, therefore, very susceptible to parasite burdens. They also are susceptible to fluke infestations. Each farm will need a specific parasite control plan in place to help minimise disease and resistance, dependent on herd size, grazing platform size and disease status.
Signs of ill thrift, weight loss, with/without diarrhoea can be common clinical signs of parasitic disease. Fly strike can be seen during the hotter months if dirty hindquarters are not dealt with correctly.
Faecal sampling is the main monitoring tool used in alpacas and samples must be collected from individual animals. Monitoring should be carried out at least twice yearly in healthy animals. Clean pasture is ideal for the most at risk animals including weanlings and cria, however not always available. Poo picking fields, as done in relation to horse pastures, is a good way to reduce parasitic burden, especially if grazing areas are limited.
Worming products typically used for alpacas include:
- Benzimidazoles (e.g. Panacur 10% oral suspension 2ml per 10kg orally and when confirmed or suspected Nematodirus/whipworm to be used for four consecutive days, can use a higher dose if Giardia is confirmed or suspected)
- Avermectins (e.g. Ivomec injection 0.6mg/kg sub-cutaneously)
- Moxidectin (e.g. Cydectin 0.4mg/kg orally)
- Coccidiosis (e.g. Baycox bovis, 3ml per 10kg orally, Vecoxan 1ml per 2.5kg orally)
- Fluke treatment Triclabendazole (e.g. Fasinex 12mg/kg orally), Closental (Flukiver 7.5mg/kg orally)
All bought in animals should be isolated from the main herd for 21 days and have a worm egg count preformed.
Vaccination
Vaccination should be carried out routinely to help protect against clostridial diseases. Most vaccines cover multiple strains of clostridial disease and include Lambivac (2ml), Heptovac (1ml), Bravoxin 10 (1ml) and Covexin 10 (1ml). There are no licenced vaccines for alpacas but the current recommended vaccine is Bravoxin 10/Covexin 10 due to the type A cover.
A primary course should be completed with two doses given at four to six weeks apart and can be started from two to three months of age if the cria are from vaccinated mothers and received sufficient colostrum. A booster vaccine should be given annually.
For pregnant females, giving a booster vaccine four to six weeks before
unpacking will ensure there are maximum numbers of antibodies in the colostrum. If the vaccination status of the dam is unknown, the cria can be given Lambivac at 48 hours old and given again at two to three weeks, before being moved over to the adult clostridial vaccine schedule with a primary course four to six weeks apart repeated annually. Animals that have not received a clostridial vaccine and have a suspicion of tetanus can be given tetanus anti-toxin (3ml maximum for an adult).
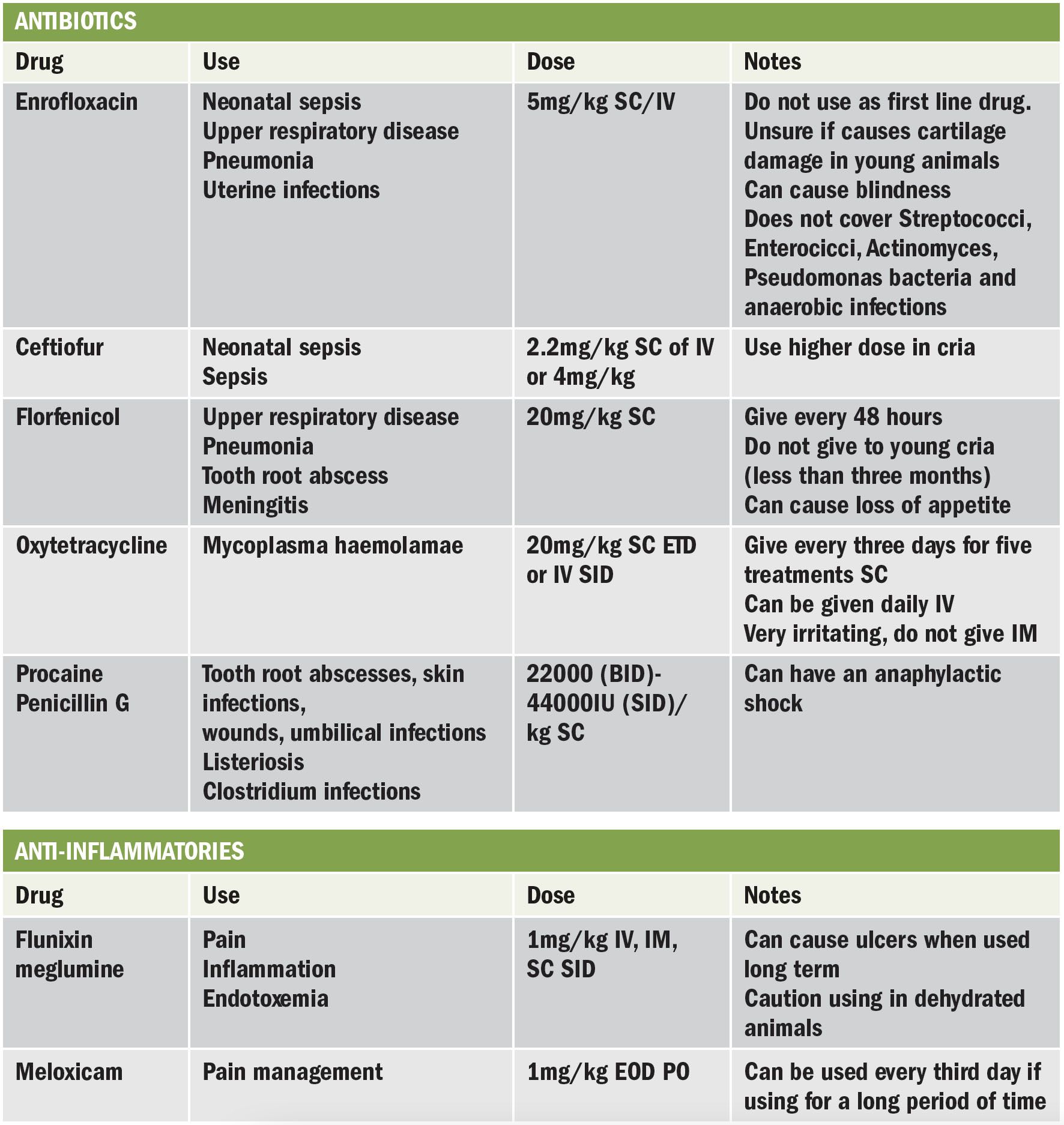
Antibiotics and anti-inflammatories
There are no licenced products for alpacas, but due to their popularity, new research is published regarding required doses.
Ulcers
Alpacas can suffer from third compartment (C3) ulceration, similar to those seen in horses and can become worse during stressful periods like illness. It is recommended to treat alpacas with IV Pantopazole (1mg/kg IV q 24hrs for three days or 2mg/kg SC q24hours for three days) or Omeprazole (0.4 and 0.8 mg/kg IV q12 hours for three days).
This helps increase the pH of the C3. Oral Omeprazole is not recommended in any animal that is old enough to chew cud as it will not pass into C3. Severely-affected ulcer patients may require a blood transfusion. In order to achieve this, a commercial blood transfusion bag can be used to collect one bag of blood from a healthy donor from the same farm (ideally a castrated, mature, quiet male) and transfuse this to the patient using a giving set with a filter.
Vitamin D
Vitamin D is produced when ultraviolet light is absorbed by the skin and is needed for healthy bone growth and skin. Alpacas in their natural South
American environment will be exposed to much higher levels of UV light compared to in Ireland. It is therefore important to supplement Vitamin D to prevent poor calcified bones, similar to those seen in human Rickets disease.
Vitamin D can be given orally (ADE paste, as directed) or by injection (Duphafral ADE, 1000IU/kg for prevention and 2000IU/kg for treatment). Younger, growing animals will require up to three doses (November, January, March) and all animals should be given one dose in November. Pregnant females should receive two doses in November and January. Some camelid-specific feeds such as Camelibra (GWF Nutrition) will also contain other vitamin and mineral supplements.
Cria Care
Cria, like calves and lambs, need to get sufficient colostrum within 24 hours of birth. If a cria has not drank since birth, it is important to check the blood glucose concentration and supplement if this is too low. Like all other neonates cria are susceptible to hypothermia and, therefore, should be provided with a coat and a heat source to warm up.
Cria that have had insufficient (or no) colostrum within six hours of birth are very susceptible to sepsis and as such IgG levels should also be checked. If IgG levels are low (below 5.5g/dl) and the cria is weak, a plasma transfusion may be needed. For small cria, one unit (300ml) of plasma tends to be sufficient but larger cria or those that are septic may require more. Plasma should be given with a giving set with a filter.
For the breeding season, it is recommended that larger farms have stored frozen plasma ready, which if stored correctly, can be kept for five years. Otherwise, it is necessary to take a unit of blood from a fully-vaccinated male on the same farm, which then has to be spun down and the serum given to the cria slowly through an IV catheter.
Sedation for castration and castration methods
Castration of males decreases testosterone production and can prevent unwanted behaviours in small groups. Gelded males are very popular as pets. Alpacas are typically castrated at approximately 18 months old, when they are fully grown, however the procedure can be performed later, dependent on husbandry and behaviour.
Pre-operative preparation
Alpacas should be fully vaccinated for tetanus and other clostridial diseases well in advance of any surgery. Food and water should be withheld 12 hours prior to the surgery to prevent regurgitation occurring. A broad-spectrum antibiotic like Penicillin (procaine penicillin 22,000 – 44,000IU/kg BID continued for five days) should be given pre-operatively as well as a non-steroidal anti-inflammatory (Flunixin meglumine 1mg/kg sub-cut).
Sedation
A heavy sedation protocol can be used to keep the alpaca standing with some support; placing a bale between the fore and hind limbs works well and allows clear visualisation of the surgical field. A combination of xylazine (0.3-0.5mg/kg, IM), butorphanol (0.05 - 0.1mg/kg, IM), and ketamine (3-5mg/kg, IM) may be used to heavily sedate the alpaca. Local anaesthetic can be given into the testicle and the skin where the incision will be on each side of the scrotum (2ml, 2 per cent lidocaine HCl each site).
Surgery
After a sterile clip and prep of the area using antiseptic surgical scrub, a 2-3cm incision can be made over the testicle on either side parallel to the median raphe along the caudal ventral most aspect of the scrotum, applying pressure to free it from the scrotum. The ligament should be gently broken and using sterile swabs and any fascia should be removed, keeping the tunic closed. Once the fascia is stripped back, a three-clamp method can be used and transfixation and then an encircling suture should be placed to ligate the spermatic cord (No 0 chromatic gut, No 2-0 polyglactin 910). Emasculation of the spermatic cord can be performed but can be difficult due to the small size. Once the testicle is removed, the spermatic cord should be gently placed back into the scrotum checking for haemorrhage. The scrotum is left open and no tissue should protrude out of the incision site. Topical antibiotic spray can be applied and fly treatment is recommended in hot weather.
In association with

Victoria Rhodes
UCD Veterinary Hospital, University College Dublin
Fay Pooley
Scarsdale Vets, Derby, United Kingdom
Marijke Beltman
UCD Veterinary Hospital, University College Dublin









