UCD Research - July 2020
Mechanisms of inflammation in primary immune-mediated haemolytic anaemia
Researchers at University College Dublin are investigating mechanisms involved in primary immune-mediated haemolytic anaemia in dogs through microRNA evaluation
In association with


Benoît Cuq Dr Vét Dipl ACVIM
Assistant professor – small animal internal medicine; American and VCI specialist in small animal internal medicine

Antoine Duclos Dr Vét
DVMS student and ECVIM resident in small animal internal medicine
Background
Immune-mediated haemolytic anaemia (IMHA) is one of the most common immune-mediated haematological disorders in dogs, resulting in life-threatening anaemia and predisposition to severe thromboembolic events (Balch and Mackin, 2007). Recently, a veterinary consensus has been published, providing useful guidelines for diagnosis and treatment of IMHA in dogs (Garden et al, 2019; Swann et al, 2019).
Compared to earlier studies, the mortality rate has been decreasing over time, probably in relation to increased awareness, earlier recognition and improved treatment modalities. However, it remains high (30% in recent studies), especially within the first two weeks of treatment (Weingart et al, 2019).
In IMHA, destruction of erythrocytes is antibody mediated. Antibodies are directed against erythrocytes, leading to intravascular haemolysis through complement activation and extravascular haemolysis through activation of mononuclear phagocyte system in the liver and spleen (McCullough, 2003).
Triggers such as infectious diseases (including tick-borne diseases), neoplasia, vaccination, inflammation and drug therapy have been associated with IMHA in dogs (Garden et al, 2019).
However, 75-93% of canine IMHA presented to referral hospitals do not have an identified underlying cause and, thus, are considered primary. Some breeds are over-represented in this disease (Miller et al, 2004).
Clinical signs of canine IMHA can vary widely in severity from mild to life threatening. According to Piek et al (2008), most common clinical signs include: weakness (93%); anorexia (80%); discoloured urine (44%); vomiting (29.5%); diarrhoea (15%); respiratory distress (10.7%); and haemorrhagic diathesis (10%). Icterus, secondary to the accumulation of bilirubin in blood and tissues, has also been reported in these patients and was significantly more frequent in non-survivor dogs (53%) than survivors (28%) in one study (Holahan et al, 2010).
Management of IMHA includes immunosuppressant therapy, antiplatelet or anticoagulant drugs for thromboprophylaxis, as well as intense supportive care including intensive care unit (ICU) hospitalisation, intra-venous (IV) fluid therapy and blood product administration. Immunosuppressant therapy is necessary to stop the destructive haemolytic process.
Mechanisms involved in primary IMHA
The exact pathogenesis remains poorly understood. For this reason, current treatments rely mainly on broad inhibition of the immune system and on management of complications (Swann et al, 2019). Broad immunosuppressants can be costly, associated with side effects and not always successful. Therefore, a better comprehension of the mechanisms involved in primary IMHA could lead to more targeted immunosuppressive therapy and improvement in the outcome.
In humans, investigations in autoimmune diseases highlighted the role of dysregulation of Th17 cells, a subtype of T-helper lymphocytes. Dysregulation of IL-17, a proinflammatory cytokine produced by Th17 cells, has been suggested as one of the key mechanism (Tabarkiewicz et al, 2015). Auto-immune haemolytic anaemia (AIHA) is a human autoimmune disease that shares some similarities with canine IMHA. Dysregulation of Th17 and IL-17 has been identified in AIHA patients. Interestingly, concentration of IL-17 has been associated with disease severity (Xu et al, 2012; Hall et al, 2012). Recently, an increase in serum IL-17 concentration was identified in dogs with primary IMHA that did not survive, compared to healthy patients (Cuq et al, 2017).
MicroRNAs are small non-coding RNAs that play a key role in the regulation of gene expression (Hammond, 2015). They are being extensively investigated in neoplastic, neurologic, hepatic and autoimmune diseases both in human and veterinary medicine (Sahabi et al, 2018). They are being pursued as clinical diagnostics as well as therapeutic targets (Hammond, 2015; Zhang et al, 2020). Notably, alteration of microRNA profile has been shown to play a key role in the dysregulation of Th17 and IL-17 in autoimmune diseases (Khan and Ansar Ahmed, 2015) and AIHA in humans (Xing et al, 2018). There have been no studies to date investigating the role of microRNAs in canine primary IMHA.
Study design
In this pilot study, Benoît Cuq and Antoine Duclos will compare microRNA profile between dogs with primary IMHA and healthy dogs through a multicentric retrospective study on dogs that underwent necropsy. The three centres involved will be UCD Veterinary Hospital and two academic referral centres in Canada. Selected microRNAs, known to be involved in alteration of Th17 and IL-17 in human autoimmune diseases, will be evaluated from the liver and spleen of dogs with primary IMHA who had a necropsy performed in these institutions.
If results are relevant, further studies can be conducted to evaluate serum concentration of microRNAs profile in dogs with primary IMHA, and compare them between healthy dogs, dogs with non-immune-mediated haemolytic anaemia and dogs with secondary immune-mediated haemolytic anaemia. These studies will be aimed at identifying selected microRNAs as potential biomarkers and therapeutic targets to improve understanding and management of canine primary IMHA.
References available on request.
Effect of radiation dose reduction on image quality and scatter radiation in equine head CT
Background
Standing computed tomography (CT) examinations of the equine head are becoming more frequently performed in clinical practice. Recently a 16-slice sliding gantry CT scanner (SOMATOM Scope, Siemens, Erlangen, Germany) has been installed at the UCD Veterinary Hospital. A range of radiation doses (mAs) are described in the literature for acquiring CT studies of the equine head. Reducing the ionising radiation dose used to acquire a CT study should reduce scatter radiation exposure to veterinary personnel. However, reducing radiation dose may compromise image quality due to increased image noise. This study aimed to:
1) Assess objectively and subjectively whether reducing mAs significantly reduced image quality for equine head examinations; and
2) Objectively assess the extent of scatter radiation reduction achieved by reducing mAs.
Materials and methods
CT examination of 10 equine cadaver heads was performed. Each head was weighed and scanned three times, at 300mAs, 225mAs and 150mAs. All other acquisition parameters remained identical. Series underwent reconstruction using medium and high frequency algorithms, assessed using a pre-determined window width and level. Image quality was assessed objectively (contrast-to-noise ratio and signal-to-noise ratio). For each acquisition, three board-certified radiologists subjectively assessed 25 anatomical structures using a four-point scale visual grade analysis. An adult human anthropomorphic phantom placed adjacent to each horse head simulated an equine handler. An ionisation chamber attached to the phantom at eye level recorded scatter radiation dose.
Results
No significant difference in image quality was identified between examinations performed at 300mAs, 225mAs nor 150mAs (Figure 1). Scatter radiation to the bystander significantly decreased with decreasing mAs and with decreasing head weight (Figure 2).
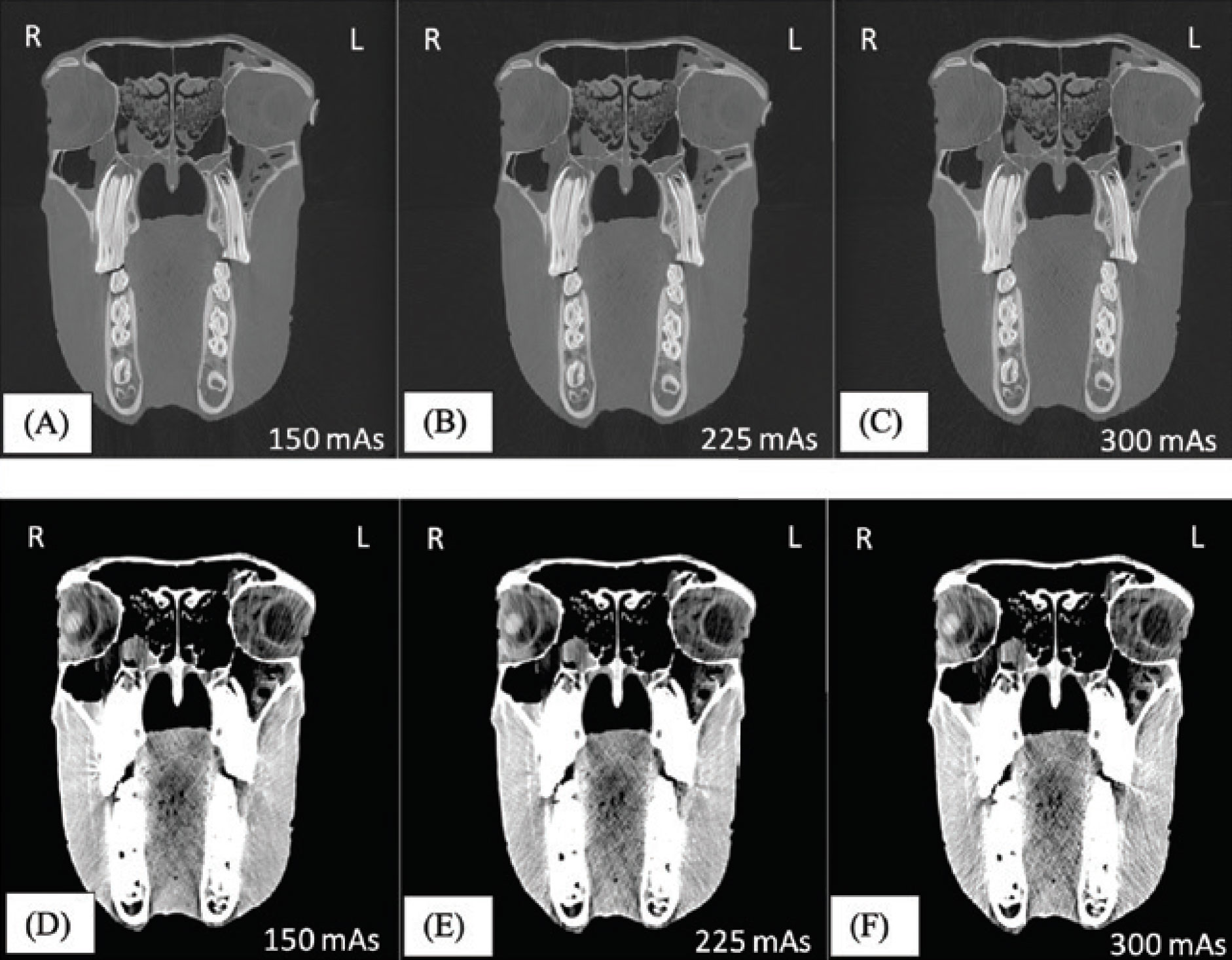
Figure 1.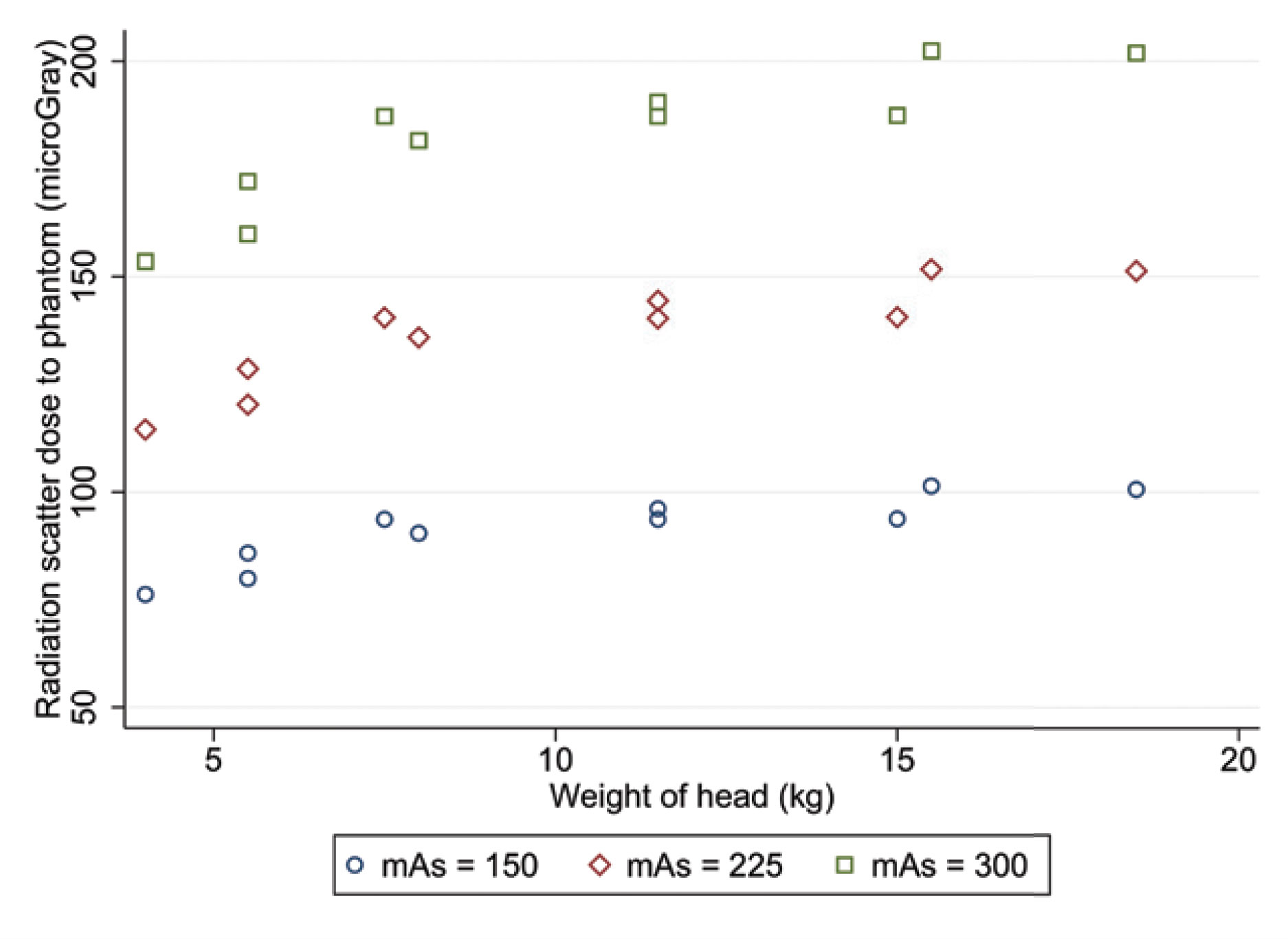
Figure 2.
Conclusion
For CT examination of the equine head, when mAs are reduced from 300mAs to 150mAs, image quality is maintained, and scatter radiation is significantly reduced.
Research team
- Thomas Davies
- Cliona Skelly
- Antonella Puggioni
- Catherine D’Helft
- Susan Connolly
- Séamus Hoey
From the Diagnostic Imaging Department, University College Dublin, Dublin, Ireland.
In association with


Benoît Cuq Dr Vét Dipl ACVIM
Assistant professor – small animal internal medicine; American and VCI specialist in small animal internal medicine

Antoine Duclos Dr Vét
DVMS student and ECVIM resident in small animal internal medicine









