Surgical management of patent ductus arteriosus in dogs
In this article, Ronan A. Mullins MVB DECVS PGDipUTL DVMS MRCVS EBVS, European specialist in small animal surgery, associate professor and discipline head of small animal surgery at University College Dublin, gives an overview of patent ductus arteriosus in dogs with a focus on surgical management
Patent ductus arteriosus (PDA) is one of the most commonly diagnosed congenital heart defects in dogs (Oliveira et al, 2011; Schrope, 2015). It occurs when the ductus arteriosus, a vessel that allows venous blood to bypass collapsed foetal lungs in utero, fails to close after birth. This results in abnormal blood flow between the descending aorta and main pulmonary artery, leading to left-sided volume overload (Pelosi and Orton, 2018). Understanding the pathophysiology, clinical manifestations, diagnostic procedures, and treatment options is crucial for ensuring appropriate intervention and management. This article provides a comprehensive overview of PDA in dogs, with a focus on surgical management.
Pathophysiology of PDA
In foetal circulation, the ductus arteriosus connects the main pulmonary artery to the descending aorta, enabling venous blood to bypass developing lungs that are not yet capable of oxygen exchange (Pelosi and Orton, 2018). At birth, increased oxygen content in the blood triggers ductus closure, which usually occurs by seven-to-10 days after birth. Eventually, the ductus transforms into a ligament, the ligamentum arteriosum (Pelosi and Orton, 2018). In cases where the ductus remains patent, left-to-right shunting of blood from the aorta to the pulmonary artery ensues due to greater left-sided pressures. This leads to severe volume overload of the left side of the heart, with subsequent left ventricular and atrial dilation, progressive myocardial deterioration, and left-sided heart failure (Pelosi and Orton, 2018).
Progressive left ventricular and atrial dilation can result in widening of the mitral valve annulus and subsequent regurgitation, leading to further volume overload of the left ventricle (Pelosi and Orton, 2018). Uncommonly, patients with PDAs can develop shunt reversal as a result of chronic pulmonary overcirculation and subsequent suprasystemic pulmonary hypertension due to irreversible lung changes. When this occurs, pulmonary arterial pressure exceeds aortic pressure and reversal of flow occurs (Eisenmenger’s syndrome).
Signalment
Patent ductus arteriosus is generally a congenital condition, inherited in many cases, although the exact cause remains unclear. It is more commonly seen in purebred dogs, with a predilection for females (Hunt et al, 2001; Van Israël et al, 2003; Bureau et al, 2005; Goodrich et al, 2007; Oliveira et al, 2011; Pelosi and Orton, 2018; Van Israël et al, 2002; Grimes and Thieman Mankin, 2022; McNamara et al, 2023; Manzoni et al, 2025). Several small and toy breeds can be affected, including Maltese, Bichon frise, Yorkshire terriers, Poodles, Pomeranians, Cavalier King Charles Spaniels, Welsh Corgi, Jack Russell terriers, and Chihuahuas, however, medium-to-large breeds such as Border Collies, Cocker and Springer Spaniels, Keeshond, Shetland Sheepdogs, Labradors, Golden Retrievers, and German Shepherd can also be affected (Hunt et al, 2001; Van Israël et al, 2003; Stanley et al, 2003; Bureau et al, 2005; Wesselowski et al, 2019; Van Israël et al, 2002; Goodrich et al, 2007; Grimes and Thieman Mankin, 2002; McNamara et al, 2023; Saunders et al, 2014; Manzoni et al, 2025).
The condition can also be seen in mixed breeds (Van Israël et al, 2002; Stanley et al, 2003; Bureau et al, 2005; Goodrich et al, 2007; Saunders et al, 2014; Grimes and Thieman Mankin, 2022). Dogs with PDA do not always present before one year of age (Van Israël et al, 2002; Van Israël et al, 2003; Saunders et al, 2014). In one study (Van Israël et al, 2002), forty percent of affected dogs were older than one year of age at initial presentation, with a mean age at diagnosis of 17 months. A heritable basis has been identified in certain breeds, in which hypoplasia and segmental asymmetry of ductal muscle mass results in failure of ductus contraction and closure.
Clinical signs
Patients with PDAs are often asymptomatic when first diagnosed (Van Israël et al, 2002, Van Israël et al, 2003; Saunders et al, 2014; McNamara et al, 2023). In one study (Van Israël et al, 2002), almost 70 per cent of dogs showed no clinical signs at the time of diagnosis. Clinical signs can include lethargy, exercise intolerance, collapse, coughing, tachypnoea, dyspnoea, and retarded growth (Stanley et al, 2003; McNamara et al, 2023; Manzoni et al, 2025).
Patients with reversed or right-to-left shunting PDAs experience hypoxemia, cyanosis (worse during exercise), polycythaemia (because of cyanosis and increased release of erythropoietin), and hyperviscosity syndrome (because of erythrocytosis). These dogs have a different collection of clinical signs, including exercise intolerance, exertional pelvic limb collapse, and possible differential cyanosis (affecting the caudal mucous membranes such as the vulva/prepuce only) (Greet et al, 2021).
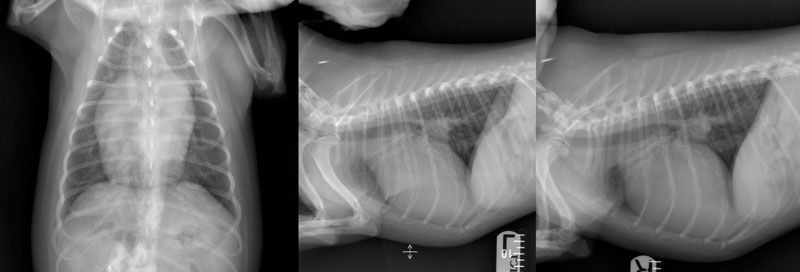
Figure 1: Preoperative dorsoventral (left), left lateral (middle), and right lateral (right) radiographs of the thorax of a 2.5-year-old female spayed Cavachon with a patent ductus arteriosus demonstrating moderate generalised cardiomegaly with focal bulging in the region of the proximal descending aorta, left atrium, and auricular appendix, and concurrent pulmonary vein congestion. There is also moderate divergence of the left and right mainstem bronchi (‘bow-legged cowboy’ sign) and moderate dorsal displacement of the thoracic trachea. Images courtesy of Dr Seamus Hoey (University College Dublin).
Diagnosis
Diagnosis of PDA requires a combination of physical examination and diagnostic imaging findings.
Physical examination and auscultation: The presence of a loud (typically grade IV-VI) continuous murmur at the left heart base (basilar) with or without continuous precordial thrill is almost pathognomonic of a PDA (Van Israël et al, 2002, Van Israël et al, 2003; Saunders et al, 2014; McNamara et al, 2023; Manzoni et al, 2025). The left-sided cardiac impulse may be displaced caudally because of cardiomegaly. Femoral pulses are bounding or hyperkinetic (“water hammer”) because of low diastolic pressure and wide pulse pressure, caused by shunting of blood through the ductus during diastole. Patients with reversed/right-to-left shunting PDAs usually will not have an audible murmur and femoral pulses will be normal.
Thoracic radiographs: Radiographs may identify signs supportive of PDA but cannot be used as a sole method of diagnosis. Typical radiographic changes include moderate to severe left-sided cardiomegaly and possibly a triple knuckle/bulge sign (Figure 1). The latter is formed by enlargement of the aorta (located at 12-1 pm), pulmonary artery (1-2 pm), and left auricle (2-3 pm) on a well-positioned dorsoventral radiograph. However, in one study (Van Israël et al, 2002), the radiographic triad of dilation of these three structures was noted in only about a quarter of cases. Elevation of the trachea, pulmonary oedema, and overperfusion of the pulmonary vasculature may also be observed (Van Israël et al, 2002; Van Israël et al, 2003).
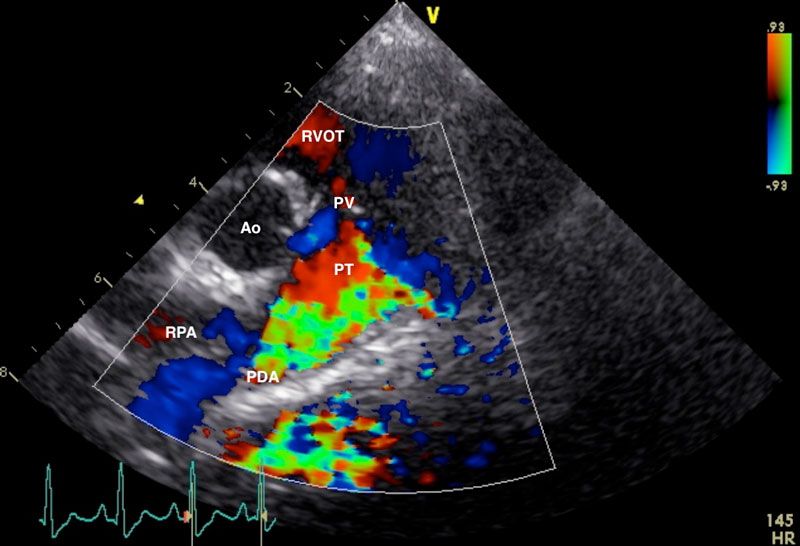
Figure 2: Right parasternal view of the aorta (Ao), right ventricular outflow tract (RVOT), pulmonary trunk (PT), and right pulmonary artery (RPA), showing a high velocity, turbulent, retrograde jet from the ductus ostium (DO) through the pulmonary trunk. Image courtesy of Dr Charlie Neill (University College Dublin).
Echocardiography: This is the gold standard for definitive diagnosis of PDA in dogs. It also allows exclusion of any concurrent cardiac defects (e.g., aortic stenosis, pulmonic stenosis, mitral dysplasia, persistent left cranial vena cava) (Van Israël et al, 2002; Van Israël et al, 2003; Saunders et al, 2014; McNamara et al. 2023). Typical findings may include left atrial and ventricular enlargement (Van Israël et al, 2002; Van Israël et al, 2003; Bureau et al, 2005; Manzoni et al, 2025), dilation of the main pulmonary artery, increased aortic ejection/outflow velocity, and turbulent flow in the pulmonary artery on doppler analysis (Figure 2). Acquired mitral regurgitation may also be identified (Van Israël et al, 2003; Bureau et al, 2005; McNamara et al, 2023; Manzoni et al, 2025).
Electrocardiography (ECG): An ECG may be performed to detect arrhythmias that might occur secondary to cardiac enlargement. Tall R waves, deep Q waves, or both, may be seen on a lead II and are supportive but not always present (Van Israël et al, 2002; McNamara et al, 2023). Tall or wide P waves can also be seen (Van Israël et al, 2002). Most dogs are in sinus rhythm or sinus arrhythmia, but atrial/ventricular premature complexes or atrial fibrillation can be seen in some cases (Stanley et al, 2003; Van Israël et al, 2002, Van Israël et al, 2003; Bureau et al, 2005; McNamara et al, 2023; Manzoni et al, 2025).
Angiography: A classification system for PDA has been described in dogs, which categorises PDAs into one of four phenotypes (types I, IIA, IIB, and III). This classification is based on factors such as degree of ductal tapering and the presence and location of abrupt ductal narrowing (Miller et al, 2006).
Treatment options
Options for management of left to right shunting PDA in dogs include open surgical ligation and minimally invasive transcatheter closure. Closure should be performed as soon as possible to avoid congestive heart failure and shunt reversal. Closure is not performed in the case of right-to-left or bidirectional PDA, with treatment aimed at management of erythrocytosis (serial phlebotomy).
Surgical closure: This surgery requires a thorough knowledge of the anatomy of the pertinent vascular, and on the basis of the risk of possible life-threatening haemorrhage, should be performed only by those with extensive surgical experience in this area. Surgical closure should be performed any time from approximately two months of age and ideally before four months of age (Pelosi and Orton, 2018). If diagnosed in older patients, closure should be performed as soon as possible. Patients with pulmonary oedema on thoracic radiographs or significant cardiomegaly and left ventricular dilation on echocardiography may require medical treatment (e.g., diuretics, pimobendan) prior to anaesthesia. Surgical closure involves a left fourth (most commonly) intercostal thoracotomy and tying off the PDA with 2-0 silk (Hunt et al, 2001; Goodrich et al, 2007; Grimes and Thieman Mankin, 2022; McNamara et al, 2023); see Figure 3. A lateral thoracic radiograph can be obtained preoperatively to determine if the left fourth or fifth intercostal space will provide best access to the root of the aorta. A rolled towel can be placed under the thorax to separate the ribs and elevate the heart. A finochietto retractor is used for rib distraction. The left cranial lung lobe is retracted and maintained caudally with a moist swab, exposing the heart. The vagus nerve typically travels over the PDA and is carefully dissected without injury and retracted dorsally or ventrally (Manzoni et al, 2025). Placing a finger over the PDA will identify a thrill. Without opening the pericardium (extrapericardial dissection), dissection of the PDA is performed cranially and caudally with a curved Mixter or Lahey forceps. Initially, dissection should progress vertically on either side of the ductus, followed by around (deep to) the PDA. Great care is taken not to injure the PDA, which could lead to life-threatening haemorrhage. Following passage of a curved forceps from caudal to cranial, a loop of 2-0 silk is grasped between the tips and retrieved caudally. The loop is then cut, creating two strands, one dorsal and one ventral. This technique eliminates the need to pass the forceps twice (Hunt et al, 2001; Manzoni et al, 2025). The suture closest to the aorta is tied first over a period of five-to-10 seconds, making sure to have the suture tight but not break it and potentially injure the ductus. Closure of the PDA can result in a Branham reflex, a decrease in heart rate secondary to an abrupt increase in diastolic pressure (Madruga et al, 2021). Following closure of the PDA, the left cranial lung lobe is returned to a normal position and a swab count performed. A MILA chest drain can be placed to evacuate pneumothorax or air can be removed by means of thoracocentesis. A left apical holosystolic murmur can be auscultated postoperatively in patients with preoperative mitral regurgitation (Bureau et al, 2005).
Endovascular/transcatheter closure: Patent ductus arteriosus closure can also be achieved using devices such as the Amplatz canine duct occluder, embolisation coils, and Amplatzer vascular plugs (Saunders et al, 2014; Grimes and Thieman Mankin, 2022). Arterial access is typically gained via a femoral artery. Minimally invasive transcatheter PDA occlusion has become commonplace in dogs. It is less invasive, carries a lower risk of haemorrhage, and is associated with a shorter recovery than thoracotomy.
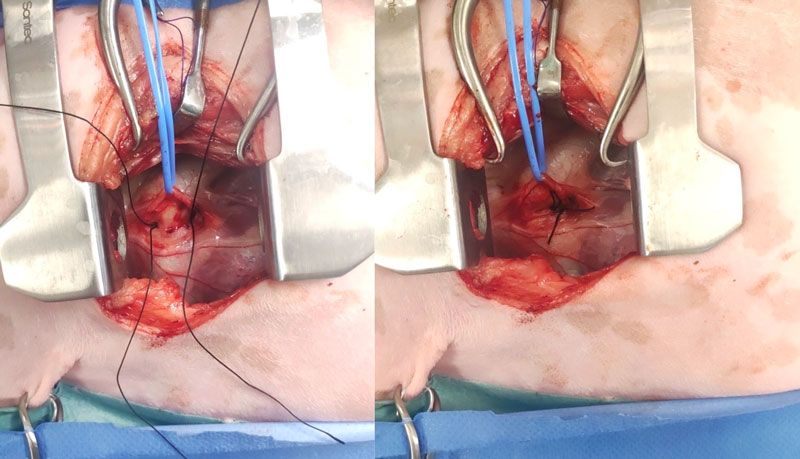
Figure 3: Left fourth intercostal space thoracotomy in a dog with a patent ductus arteriosus. The vagus nerve has been carefully dissected and gently retracted dorsally using a blue vessel loop. A single loop of 2-0 silk has been passed from cranial to caudal medial to the ductus and cut, creating two single strands (left). Starting with the dorsal strand closest to the aorta, both strands are tied slowly and securely, taking care not to break the suture or injure the ductus (right). Cranial is to the left of the images.
Complications
One of the biggest risks with surgical closure is injury to the ductus and occurrence of potential life-threatening haemorrhage (Hunt et al, 2001; Bureau et al, 2005; Goodrich et al, 2007; Grimes and Thieman Mankin, 2022; McNamara et al, 2023; Manzoni et al, 2025). When haemorrhage does occur, the rate of survival is very variable, ranging from zero per cent to 100 per cent (Hunt et al, 2001; Stanley et al, 2003; Bureau et al, 2005; Goodrich et al, 2007). However, in one of the most recent large-scale studies, seven per cent experienced haemorrhage and all survived (Grimes and Thieman Mankin, 2022). The risk of haemorrhage has been shown in older studies to decrease with surgeon experience and increase in older animals (Hunt et al, 2001; Bureau et al, 2005).
It has been hypothesised that more organised and fibrous connective tissue around the ductus with increasing age is associated with more difficult dissection in older animals (Hunt et al, 2001). Two recent retrospective studies involving 285 dogs and 417 dogs, respectively, reported a 7.0 to 10.8 per cent rate of PDA rupture (Grimes and Thieman Mankin, 2022; McNamara et al, 2023). In one of these studies (Grimes and Thieman Mankin, 2022), there was no difference in the age of dogs with or without rupture.
Other possible complications include persistence of flow through the ductus postoperatively (Van Israël et al, 2002; Van Israël et al, 2003; Stanley et al, 2003; Goodrich et al, 2007; Grimes and Thieman Mankin, 2022; McNamara et al, 2023), recanalisation of the ductus (Van Israël et al, 2003), postoperative laryngeal paralysis (Van Israël et al, 2002; Van Israël et al, 2003; Manzoni et al, 2025), lung lobe injury (Van Israël et al, 2002; Goodrich et al, 2007), cardiac/cardiopulmonary arrest (Van Israël et al, 2002; Van Israël et al, 2003; Grimes and Thieman Mankin, 2022; McNamara et al, 2023), respiratory insufficiency requiring ventilatory support (Goodrich et al, 2007), haemothorax (McNamara et al, 2023), chylothorax (Van Israël et al, 2002, Goodrich et al, 2007), wound seroma (Van Israël et al, 2002; Van Israël et al, 2003), pneumothorax (Van Israël et al, 2002), and inadvertent ligation of the main pulmonary artery or one of its branches (Grimes and Thieman Mankin, 2022). A recent large-scale study reported a 9.4 per cent rate of residual flow with ligation (Grimes and Thieman Mankin, 2022). In some cases, residual postoperative flow through the ductus can resolve spontaneously, whereas in others, a second surgical procedure to ligate the PDA may be required (Grimes and Thieman Mankin, 2022). In one study (Grimes and Thieman Mankin, 2022), dogs that had experienced rupture of their PDA were more likely to have residual flow than dogs without rupture. Finally, anaesthetic complications are commonly observed and can include hypothermia, bradycardia, and hypotension (McNamara et al, 2023).
Prognosis
The prognosis for most dogs that undergo treatment of their PDA is generally good. Closure of the ductus improves the prognosis (Saunders et al, 2014), and is indicated in all dogs with left-to-right PDA, even in those with left-sided congestive heart failure or secondary functional mitral regurgitation (Pelosi and Orton, 2018). This is because of the poor long-term prognosis without correction, with 64 per cent of unoperated dogs dying within 12 months in one study (Eyster et al, 1976). With successful closure, resolution of clinical signs can also be observed in the majority of older dogs (Van Israël et al, 2003). The only contraindication to closure is if reversal of the PDA (right-to-left or bidirectional shunting) has occurred. The rate of survival to discharge in two large retrospective studies that included a total of 702 dogs was 97.0 to 99.6 per cent (Grimes and Thieman Mankin, 2022; McNamara et al, 2023). One- and five-year survival rates in the largest study to date was 96.4 per cent and 87.1 per cent, respectively (McNamara et al, 2023).
There is controversy in the literature regarding the prognostic significance of certain variables such as age at the time of surgery (e.g., >24 months), bodyweight at the time of surgery (e.g., >23 kg), preoperative mitral regurgitation, preoperative atrial fibrillation, and so forth. While some studies have identified these to be negative prognostic indicators (Eyster et al, 1976), others have not (Goodrich et al, 2007; McNamara et al, 2023; Bureau et al, 2005), and these should not be considered contraindications to surgery. Finally, the prognosis for bidirectional or right-to-left shunting PDAs is variable (Greet et al, 2021). In one study (Greet et al, 2021), the median survival time of such dogs was 626 days (range 1 to 3,628 days); however, it was shorter in those with right-sided congestive heart (58 days vs 1,839 days in dogs without).
Bureau S, Monnet E, Orton EC: Evaluation of survival rate and prognostic indicators for surgical treatment of left-to-right patent ductus arteriosus in dogs: 52 cases (1995-2003). J Am Vet Med Assoc. 2005;227(11):1794–1799.
Eyster GE, Eyster JT, Cords GB, Johnston J. Patent ductus arteriosus in the dog: characteristics of occurrence and results of surgery in one hundred consecutive cases. J Am Vet Med Assoc. 1976;168(5):435-8.
Goodrich KR, Kyles AE, Kass PH, et al: Retrospective comparison of surgical ligation and transarterial catheter occlusion for treatment of patent ductus arteriosus in two hundred and four dogs (1993-2003). Vet Surg. 2007;36(1):43–49.
Grimes JA, Thieman Mankin KM. Surgical ligation of patent ductus arteriosus in dogs: Incidence and risk factors for rupture. Vet Surg. 2022;51(4):592-599.
Hunt GB, Simpson DJ, Beck JA, et al. Intraoperative hemorrhage during patent ductus arteriosus ligation in dogs. Vet Surg. 2001;30(1):58–63.
Madruga FL, Pereira YM, Panti A, Handel I, Culshaw G. Branham sign in dogs undergoing interventional patent ductus arteriosus occlusion or surgical ligation: A retrospective study. Open Vet J. 2021;11(4):603-612.
Manzoni S, Van Israël N, Santos M, and Bongartz A. Effects of left vagus nerve retraction on post-operative unilateral left-sided laryngeal paralysis following surgical closure of patent ductus arteriosus in dogs. J Small Anim Pract. 2025. doi: 10.1111/jsap.13867. Online ahead of print.
McNamara KL, Regier PJ, Toth D, Mickelson M, Luther J, Pyne C, Wallace M, Sturkie C, Dugat D, Marvel S, Sumner J, Scharf V, Clark J, Colee JC. Risk factors for intraoperative hemorrhage and perioperative complications and short- and long-term outcomes during surgical patent ductus arteriosus ligation in 417 dogs. J Am Vet Med Assoc. 2023;261(8):1-7.
Miller MW, Gordon SG, Saunders AB, Arsenault WG, Meurs KM, Lehmkuhl LB, Bonagura JD, Fox PR. Angiographic classification of patent ductus arteriosus morphology in the dog. J Vet Cardiol. 2006;8(2):109-14.
Oliveira P, Domenech O, Silva J, et al: Retrospective review of congenital heart disease in 976 dogs. J Vet Intern Med.25(3):477–483, 2011.
Saunders AB, Gordon SG, Boggess MM, Miller MW. Longterm outcome in dogs with patent ductus arteriosus: 520 cases (1994-2009). J Vet Intern Med. 2014;28(2):401–410.
Schrope DP. Prevalence of congenital heart disease in 76,301 mixed-breed dogs and 57,025 mixed-breed cats. J Vet Cardiol. 2015;17(3):192-202.
Stanley BJ, Luis-Fuentes V, Darke PG. Comparison of the incidence of residual shunting between two surgical techniques used for ligation of patent ductus arteriosus in the dog. Vet Surg. 2003;32(3):231-7.
Van Israël N, French AT, Dukes-McEwan J, Corcoran BM. Review of left-to-right shunting patent ductus arteriosus and short term outcome in 98 dogs. J Small Anim Pract. 2002;43(9):395-400.
Van Israël N, French AT, Dukes-McEwan J, Welsh EM. Patent Ductus Arteriosus in the older Dog. J Vet Cardiol. 2003;5(1):13-21.
Wesselowski S, Saunders AB, Gordon SG. Anatomy, baseline characteristics, and procedural outcome of patent ductus arteriosus in German Shepherd dogs. J Vet Intern Med. 2019;33(2):471-477.
The ductus arteriosus typically closes within which specific timeframe after birth in dogs?
- 1 hour
- 24 hours
- 10 days
- 1 month
2. Patent ductus arteriosus typically leads to which of the following cardiac changes?
- Left-sided pressure overload
- Left-sided volume overload
- Right-sided pressure overload
- Right-sided volume overload
3. Which of the following murmurs is most consistent with a patent ductus arteriosus in the dog?
- Continuous
- Diastolic
- End systolic
- Holosystolic
4. Dogs with patent ductus arteriosus can have a “triple knuckle/bulge” sign visible on a dorsoventral radiograph of the thorax, which is characterised by enlargement of which three structures?
- Main pulmonary artery, left auricle and left ventricle
- Thoracic aorta, main pulmonary artery and left auricle
- Thoracic aorta, main pulmonary artery and right auricle
- Thoracic aorta, right atrium and main pulmonary artery
5. What nerve travels over the patent ductus arteriosus on the left side and can be used to aid its identification at surgery?
- The phrenic nerve
- The pararecurrent laryngeal nerve
- The recurrent laryngeal nerve
- The vagus nerve
ANSWERS: 1C; 2B; 3A; 4B; 5D.
















