Surgical management of feline meningiomas
Kristina Healy MVB, surgical intern, MyVet Referrals (under the guidance of Ciarán Jones MVB MS DECVS DACVS-SA and Javier Rincon Alvarez LdoVet MVM DECVS MRCVS) discusses the surgical management of feline meningioma
Meningiomas are the most commonly diagnosed primary intracranial tumours in cats. Although typically benign, these tumours can cause significant neurological impairment due to their space-occupying nature. Surgical intervention is considered the most effective treatment, offering longer survival times compared to other therapies.
This case report illustrates the successful management of a feline meningioma through surgical excision, highlighting the importance of advanced imaging, vigilant perioperative care, and long-term follow-up in optimising surgical outcomes.
Background
Primary brain tumours are relatively uncommon in cats, with an incidence rate of approximately 2.2 per cent.6,7 Among these, meningiomas are the most frequently diagnosed primary intracranial tumours, accounting for 58 per cent of cases.1,2,6 Feline intracranial meningiomas are generally benign, slow-growing tumours originating from the arachnoid cells of the meninges.1,2,3 These tumours are typically well-defined masses, often located peripherally within the cranium, and are separated from surrounding brain tissue.7,11 In cats, meningiomas exhibit a strong predilection for the rostrotentorial region of the brain (92.4 per cent of cases), particularly involving areas such as the third ventricle and the parietal and temporal lobes.3,4,6 Meningiomas are typically solitary in cats, but multiple meningiomas may represent up to 17 per cent of cases.1,7 Extra-neural metastases are very uncommon with feline meningiomas.3
Meningiomas most commonly occur in older cats, with an average age at diagnosis being 11 to 12 years.1,2,6 There is no significant sex predilection,6 and while meningiomas are more commonly diagnosed in domestic shorthair cats, this likely reflects the high prevalence of this breed in the general cat population rather than a breed-specific predisposition.1,3
Despite their benign nature, feline meningiomas can cause significant neurological deficits due to intracranial expansion.4 This growth compresses the brain, potentially leading to cerebral oedema, and in severe cases, brain herniation.4 Clinical signs associated with feline meningiomas are often progressive and can include altered mentation, circling, seizures, and blindness.2,6 11 The increasing availability of advanced imaging technologies such as magnetic resonance imaging (MRI) and computed tomography (CT) has greatly improved the ability to diagnose intracranial tumours in cats. The sensitivity of MRI to correctly identify meningiomas is estimated to be 96 per cent in cats.12 However, while imaging provides valuable information about tumour location and characteristics, a definitive diagnosis of meningioma requires histopathological examination.3
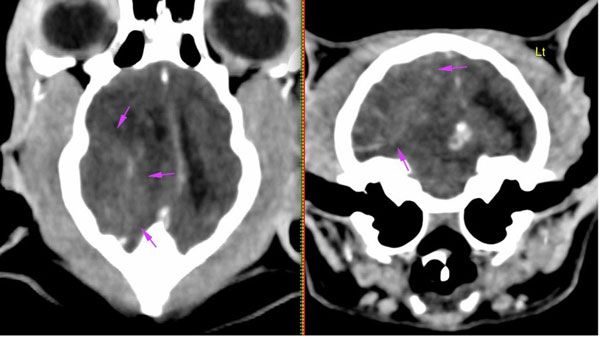
Figure 1: CT scan of the brain showing an extra-axial soft tissue mass located in the right caudodorsal part of the cranial fossa.
Treatment options for feline meningiomas vary, with surgical intervention considered the most effective approach.1 Surgery offers significantly longer survival times compared to other treatments.3 Unlike canine meningiomas, which tend to be invasive, feline meningiomas are usually non-invasive.3 Their peripheral location and well-defined nature make them more amenable to complete surgical resection.6 When feasible, surgical excision is the preferred treatment choice,1 with a median survival time of approximately 22 months following surgery.7
Occasionally, due to the location and size of the tumour, or patient comorbidities, surgery may not be a viable treatment option.7 Alternative treatments include radiation therapy and palliative care, with or without chemotherapy. Cats treated with radiation therapy have a median survival time of 14 months,1,10 while those managed with conservative treatments alone face a much shorter median survival time of only 18 days.2,3,6
Case presentation
A nine-year-old female neutered domestic short hair presented with a one-month history of progressive behavioural changes. The owner reported episodes of disorientation, circling to the right, and apparent blindness. Physical examination was unremarkable; however, neurological assessment revealed an absent menace response in both eyes, while pupillary light reflexes remained intact bilaterally. No other cranial nerve abnormalities were detected, and ophthalmic examination was normal. Based on these findings, the patient was diagnosed with central blindness, and a forebrain lesion with right-sided lateralisation suspected. After discussing potential differential diagnoses and associated risks, the patient was admitted for further diagnostic testing.
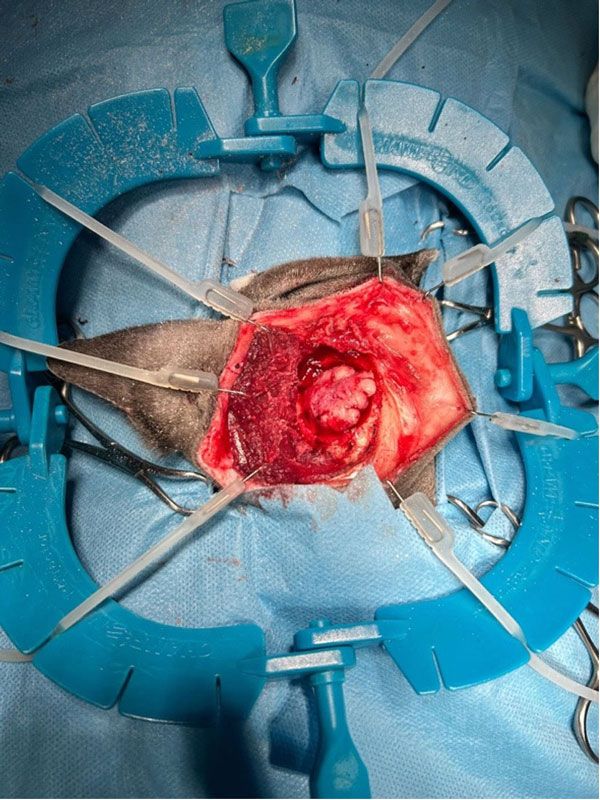
Figure 2: The meningioma in situ, dissected prior to surgical excision.
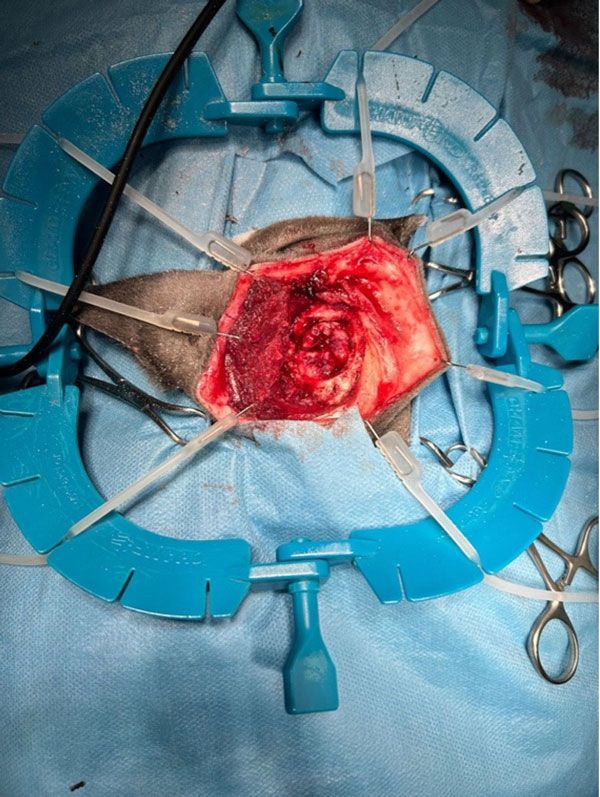
Figure 3: The meningioma in situ, dissected prior to surgical excision.
Diagnostic procedures
Blood work, including a complete blood count, and serum biochemistry yielded normal results. Blood pressure readings, urinalysis, and thyroxine levels were also within reference values. The patient underwent a computed tomography (CT) scan of the head under general anaesthesia, which revealed a moderately-sized soft tissue mass (measuring approximately 2cm x 1.35cm x 1.35cm) occupying the extra-axial space in the right caudodorsal part of the cranial fossa, at the level of the parietal, temporal, and occipital lobes. The lesion exerted a significant mass effect, causing a leftward midline shift and compression of the right lateral ventricle. Additionally, there was evidence of cerebellar herniation through the foramen magnum. Given these findings and owing to a high suspicion of meningioma and with the owner’s consent, the decision was made to proceed with surgical management of the intracranial mass. Meanwhile, the patient was started on oral prednisolone (1mg/kg, once daily) for a short period of time, resulting in partial resolution of clinical signs.
Potential complications of craniotomy/craniectomy
Surgical management of intracranial tumours in companion animals, including cats and dogs carries risks of potential perioperative complications. During the craniotomy itself, potential complications which can be encountered include: brain oedema, intraoperative haemorrhage, and cardiopulmonary dysfunction, especially in cases involving brainstem surgery.5 Post-operative complications often arise in the early post-operative period.1 These may include neurological deterioration, seizures, anaemia, and aspiration pneumonia.1,7 Each of these complications can have significant impacts on recovery, necessitating close monitoring and supportive care in the perioperative period to optimise outcomes and address any issues promptly.
Anaesthetic protocol
In preparation for general anaesthesia, the patient was administered intravenous dexamethasone (0.1mg/kg) and mannitol (1g/kg over 20 minutes) to minimise brain oedema and inflammation during surgery. Since the patient had no history of seizure activity related to the intracranial mass, prophylactic anti-epileptic medication was deemed unnecessary at this stage. For premedication, dexmedetomidine (3μg/kg) and methadone (0.3mg/kg) were administered intravenously producing a mild sedative effect. General anaesthesia was induced with propofol (2mg/kg) intravenously, ensuring a smooth induction and intubation process. Once intubated, anaesthesia was maintained using inhalant isoflurane and oxygen. Prophylactic perioperative antibiotics were initiated with cefuroxime (20mg/kg intravenously every 90 minutes during surgery) followed by oral amoxicillin-clavulanate (15mg/kg twice daily) post-operatively. To provide additional analgesia, ketamine was administered via constant rate infusion (CRI) throughout the surgery and continued for 24 hours post-operatively. Intravenous fluid support was provided using compound sodium lactate at a rate of 5ml/kg/hour during the perioperative period. Anaesthetic monitoring was rigorous, with particular focus on maintaining PaCO2 levels within reference range. Hypercapnia in anaesthetised patients can lead to increased intracranial pressure, posing a considerable risk during a craniotomy procedure.5 To mitigate this, the patient was mechanically ventilated for the duration of the surgery.
Surgical procedure
Prior to surgery, computed tomography (CT) images were carefully reviewed to plan the surgical approach and determine the optimal craniotomy site. Once the surgical site was identified, the patient was positioned in sternal recumbency with the head slightly elevated. The surgical site was aseptically prepared, and a right-sided lateral rostrotentorial approach was performed to access the intracranial mass. A midline incision was made on the head starting from the level of the lateral canthi and extending a few centimetres caudal to the external occipital protuberance. The cutaneous coli muscles were incised, and the cervicoscutularis muscle was transected along the midline. The temporalis fascia was then incised, and the temporalis muscle was reflected ventrally to expose the skull using a periosteal elevator. Using a high-speed spinal burr and Kerrison rongeurs, a craniectomy was performed, removing a portion of the calvarium overlying the tumour. The soft tissue mass was carefully isolated from the surrounding brain tissue and dissected free using bipolar cautery and sterile Q-tips, minimising trauma to adjacent structures. Fortunately, no complications were encountered during the surgery. To manage haemostasis and prevent blood clot formation on the exposed brain tissue, Surgicel (Ethicon Inc) was applied to the surgical site.13 Following tumour removal, the area was thoroughly lavaged with warmed sterile sodium chloride to remove any remaining tumour cells, to reduce the risk of recurrence. The temporalis muscle was then used to close the craniectomy defect, providing additional protection to the exposed area. Routine closure of the subcutaneous tissue and skin was performed to complete the surgery. The excised tumour mass was preserved and submitted for histopathological examination.
Post-operative management
The patient was monitored intensively following surgery. Vital parameters were assessed regularly, and despite a smooth anaesthetic recovery, the patient exhibited neurological deterioration 24 hours later. The patient showed signs of mental dullness, nystagmus, and a Cushing’s reflex, indicating elevated intracranial pressure. The patient was managed with mannitol (1g/kg) and dexamethasone (0.1mg/kg) intravenously, which resolved the neurological signs. By 48 hours post-surgery, the patient was alert, ambulatory, and eating. The post-operative medication regimen included methadone at a dose of 0.3mg/kg administered intravenously every four hours for pain management. Ketamine was given as a constant rate infusion (CRI) for 24 hours following surgery. Dexamethasone at 0.1mg/kg was administered intravenously once daily to control inflammation and reduce the risk of cerebral oedema. Gabapentin at a dose of 10mg/kg, was given orally twice daily to provide additional analgesia, particularly for neuropathic pain. Additionally, amoxicillin-clavulanate was administered at 15mg/kg orally twice daily to prevent potential infections at the surgical site. A tapering dose of oral prednisolone (starting at 1mg/kg) was also prescribed for one month post-operatively. The patient continued to respond well to treatment, exhibiting no additional complications, and was successfully discharged four days after surgery. Two weeks post-operatively the patient was bright, alert, ambulatory, and had regained full vision. By the three-month follow-up, the patient had achieved complete recovery, with full resolution of all preoperative neurological deficits.
Histopathology
The histopathology report revealed a moderately demarcated multinodular mass composed of spindle-shaped cells forming bundles and whirling structures, in a collagenous stroma with mild to moderate anisokaryosis and multifocal sparse mineralisation. The histopathological findings were consistent with a meningioma.
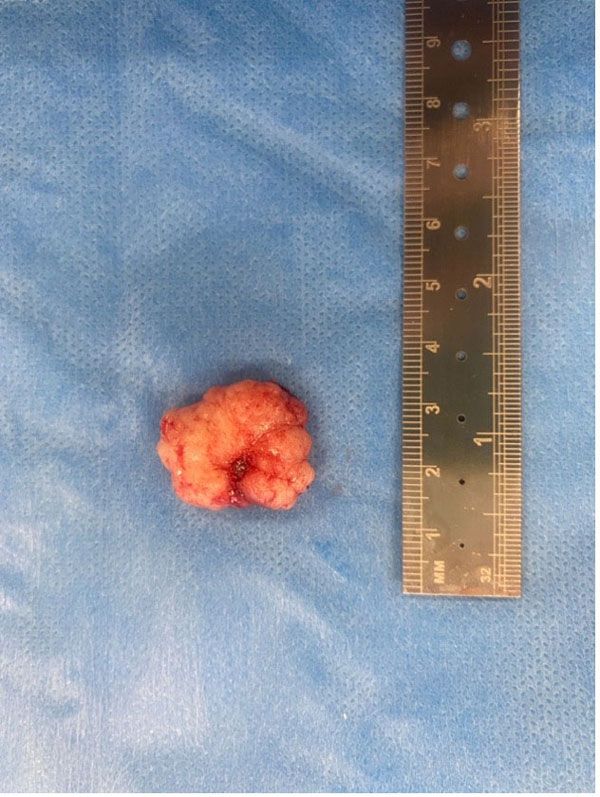
Figure 4: Meningioma.
Discussion
This case report highlights the benefits and challenges associated with the surgical management of feline intracranial meningiomas. The successful outcome demonstrates that surgery can be an effective and potentially curative treatment option when complete resection is achievable.
Surgical excision is considered the preferred treatment option for feline meningiomas, primarily due to the tumour’s well-defined borders, which facilitate complete excision while minimising the risk of damage to surrounding brain tissue. This characteristic distinguishes feline meningiomas from their canine counterparts, which are more likely to infiltrate adjacent brain tissue, complicating surgical intervention.3 In this patient, the rapid recovery of neurological function, including the resolution of vision deficits within two weeks, reinforces the effectiveness of surgical treatment of meningiomas in cats.
Studies show that cats treated surgically for meningiomas experience a significantly longer median survival time of 22 months, with a two-year survival rate of 50 per cent, compared to those treated with alternative modalities such as radiation or chemotherapy.2,7
Advanced imaging modalities, such as computed tomography (CT) and magnetic resonance imaging (MRI), play a crucial role in the diagnosis and surgical planning of intracranial meningiomas. In this case, CT imaging provided detailed tumour localisation and measurements, enabling precise surgical planning. MRI, with its superior soft-tissue contrast, can further enhance preoperative assessments, particularly in cases where distinguishing tumour margins or evaluating brain involvement is more complex. The use of advanced imaging not only supports improved diagnostic accuracy but also facilitates targeted surgical approaches and helps anticipate intraoperative challenges, ultimately enhancing surgical outcomes.1
Although surgery offers significant benefits, it is not without its challenges. The recurrence rate for feline meningiomas ranges from 11-39 per cent within three to 60 months post-operatively, with recurrences typically occurring at the original tumour site.1,8 While repeat surgeries can achieve comparable outcomes to the initial surgery, they may carry additional risks depending on the patient’s age and health status.8 This emphasises the importance of ongoing monitoring and the potential role of adjunctive therapies, such as radiation or chemotherapy.7 Further studies are needed to determine the efficacy of these complementary treatments in conjunction with surgery in managing residual or recurrent feline meningiomas.7, 3
Neurosurgical procedures involving the brain carry inherent risks and potential complications. Effective perioperative management is critical for optimising surgical outcomes. Complications are most likely to arise in the early post-operative period, necessitating vigilant monitoring of the patient.1 In this case, the patient experienced transient neurological deterioration post-operatively, which was managed effectively with dexamethasone and mannitol, enabling a marked recovery by the fourth post-operative day. This highlights the importance of proactive monitoring and timely intervention during the recovery period.
The decision to perform a cranioplasty following tumour excision remains a subject of debate in veterinary neurosurgery. While cranioplasty may be beneficial in cases with significant brain exposure or cosmetic considerations,5 in this case, the bone flap was not replaced over the craniectomy site due to concerns about potential tumour recurrence and infiltration. Although there are few reports investigating cranioplasty reconstruction in cats, in human patients undergoing brain tumour resection, cranioplasty is typically performed with either allografts or with synthetic materials to avoid tumour recurrence.1 While cranioplasty with synthetic materials may offer benefits such as preserving cerebral blood flow, the risk of infection associated with certain materials, such as polymethylmethacrylate (PMMA), as observed in human studies, must also be carefully considered.1
In conclusion, this case demonstrates both the effectiveness and the complexities of surgical management for feline intracranial meningiomas. The integration of advanced imaging techniques for preoperative surgical planning and vigilant perioperative management are vital to the success of surgical interventions. While complete surgical excision can lead to significant improvements in neurological function and extended survival times, the possible risk of tumour recurrence highlights the need for long-term monitoring and further research into adjunctive therapies. By combining surgical expertise with ongoing advancements in veterinary research, we can continue to enhance the prognosis and quality of life for cats affected by this condition.
- Porsmoguer,C., Blondel, M., Moissonnier,P. Surgical treatment of feline intracranial meningiomas: a retrospective study of 26 cases. J Vet Sci. [internet]. 2024 Mar. [cited 2024 November 12]. 25(2).: 25.1-25.12. Available from:
https://pmc.ncbi.nlm.nih.gov/articles/PMC10990911/ - Troxel MT, Vite CH, Van Winkle TJ, Newton AL, Tiches D, Dayrell-Hart B, et al. Feline intracranial neoplasia: retrospective review of 160 cases (1985-2001) J Vet Intern Med. 2003;17(6):850–859. Available from: [DOI] [PubMed]
- Motta L, Mandara MT, Skerritt GC. Canine and feline intracranial meningiomas: an updated review. Vet J. [Internet] 2012;192(2):153–165. Available from: https://www.sciencedirect.com/science/article/abs/pii/S1090023311003935?via%3Dihub
- Karli,P., Gorgas,D., Oevermann, A and Forterre, F. Extracranial expansion of a feline meningioma. J Feline Med Surg. [Internet]. 2013 Jan. [cited 2024 November 10]. 15(8): 749-53. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11191713/
- Fossum, TW, Welch TL, Duprey LP, Pardi,LA. Small animal surgery, 5th ed. Philadelphia (PA); Elsevier.;2019. [Cited 2024 November 5th] Available from:
https://go.exlibris.link/MzjnT7w4 - Cameron, S., Rishniw,M., Miller,A., Sturges,B and Dewey.,C. Characteristics and Survival of 121 Cats Undergoing Excision of Intracranial Meningiomas. (1994-2011). Veterinary Surg. [Internet]. 2015 Jun. [cited 2024 November 10] . 44(6); 772-776. Available from: https://onlinelibrary-wiley-com.ucd.idm.oclc.org/doi/10.1111/vsu.12340
- Tichenor, M., Hearon,K and S,L. Characteristics and outcomes for 61 cats that underwent either surgery or stereotactic radiotherapy as treatment for intracranial meningioma. (2005-2017). J Am Vet Med Assoc [Internet]. 2023 Jul [cited 2024 November 5]; 262(1). Available from: https://doi-org.ucd.idm.oclc.org/10.2460/javma.23.02.0083
- Johnston, SA, Tobias.KM. Veterinary Surgery (Small animal). Saunders;2017. Available from:
https://go.exlibris.link/kJFvdvND - Forterre F, Fritsch G, Kaiser S, Matiasek K, Brunnberg L. Surgical approach for tentorial meningiomas in cats: a review of six cases. J Feline Med Surg. 2006;8(4):227–233. Available from:
https://pubmed.ncbi.nlm.nih.gov/16600654/ - 11.Körner M, Roos M, Meier VS, Soukup A, Cancedda S, Parys MM, et al. Radiation therapy for intracranial tumours in cats with neurological signs. J Feline Med Surg. 2019;21(8):765–71. Available from:
https://pubmed.ncbi.nlm.nih.gov/30339060/ - Sessums K, Mariani C. Intracranial meningioma in dogs and cats: a comparative review. Compend Contin Educ Vet. 2009;31(7):330–339. Available from:
https://pubmed.ncbi.nlm.nih.gov/19746352/ - Troxel MT, Vite CH, Massicote C, McLear RC, Van Winkle TJ, Glass EN, Tiches,D, Dayrell-Hart B. Magnetic resonance imaging features of feline intracranial neoplasia. Retrospective analysis of 46 cats. J Vet Intern Med. 2004;18: 176-189. Available from:
https://pubmed.ncbi.nlm.nih.gov/15058768/ - Surgicel Original absorbable haemostat. Ethicon Inc.
1. What is the incidence rate of primary brain tumours in cats?
A. 1.5 per cent
B. 2.2 per cent
C. 3.8 per cent
D. 5 per cent
2. What type of primary intracranial tumour is most commonly diagnosed in cats?
A. Astrocytomas
B. Gliomas
C. Meningiomas
D. Pituitary tumours
3. Where do feline meningiomas most commonly occur in the brain?
A. Cerebellum
B. Rostrotentorial region
C. Brainstem
D. Spinal cord
4. What is the median survival time for cats undergoing surgical excision for meningiomas?
A. six months
B. 12 months
C. 14 months
D. 22 months
5. What is the primary method required for definitive diagnosis of feline meningiomas?
A. Computed tomography (CT)
B. Magnetic resonance imaging (MRI)
C. Histopathological examination
D. Biochemical analysis
ANSWERS: 1B;2C;3B;4D;5C.














