Meningoencephalomyelitis of Unknown Origin in Dogs
Ava Patterson, Final Year MVB Student, University College Dublin, provides an overview of canine Meningoencephalomyelitis of Unknown Origin (MUO)
The term ‘Meningoencephalomyelitis of Unknown Origin’- (MUO) comprises a collection of sporadic, idiopathic, non-infectious inflammatory brain conditions of notably young to middle-aged, small breed dogs9. MUO can have dramatic clinical presentations which prove upsetting for owners and present both diagnostic and treatment challenges for veterinarians that further complicates the clinical picture. This article will provide a review of recent literature to practically guide clinicians with diagnosis, treatment, and patient outcome.
Nomenclature
The term ‘Meningoencephalomyelitis of Unknown Origin’ (MUO) indicates a collection of sporadic, idiopathic, non-infectious inflammatory brain conditions of notably young to middle-aged, small breed dogs9. This terminology encompasses several clinically indistinct, but pathologically distinct diseases of the CNS: Granulomatous Meningoencephalomyelitis (GME), Necrotising Meningoencephalomyelitis (NME) or ‘Pug dog encephalitis’, and Necrotising Leukoencephalitis (NLE). It specifically excludes steroid responsive meningitis, idiopathic tremor syndrome, and eosinophilic meningoencephalomyelitis, as those conditions have distinct characteristics5,11.
Aetiology
While the true pathogenesis of MUO is uncertain, in a review article published by O’Neill et al (2005) several aetiologies were discussed such as infectious, use of deworming products, vaccines and neoplasia21. However, there is strong evidence that the condition is immune mediated12,21. It has been demonstrated that inflammatory cells within GME lesions, a sub-classification of MUO, consisted predominantly of MHC Class II antigen-positive macrophages and CD3 antigen positive lymphocytes which is suggestive of a T-cell mediated delayed hypersensitivity reaction12,25 . However, the causative antigen has not been identified25. Furthermore, another study identified the presence of an autoantibody against canine brain tissue in the cerebrospinal fluid (CSF) of two pugs diagnosed with another sub-classification of MUO: NME, further supporting the suspected immune mediated aetiology27.
Molecular investigations suggested a strong link between specific dog leukocyte (DLA) class II genotypes and autoimmune disorders with NME being included in this cohort10,22. A recent study investigating the correlation between high risk short tandem repeat (STR) testing and NME risk, reported that 38 to 39 of the 54 NME affected Pugs were homozygous for the predominant STR alleles and only 18 of the 229 healthy pugs were homozygous for the same alleles22 meaning that homozygous animals were at a higher risk of developing NME suggesting that genetics have an important role. As the aetiology of MUO is so ambiguous, the implementation of widespread genetic testing may be a reasonable option for attempting to reduce disease incidence going forward.
Pathology
Pathologically, the three subtypes of MUO (GME, NME and NLE) are solely distinguishable based on histological features described as follows:
GME lesions are usually located in the white matter and are characterised by perivascular accumulations of mononuclear cells consisting of lymphocytes and macrophages which are arranged in a whorling pattern21,27.
NME usually affects the grey matter but includes necrotic lesions involving the leptomeninges, cerebral cortex, corona radiata, and subcortical white matter that consist of lymphocytes, plasma cells and histiocytes29. Lesions are most common in the cerebrum but have also been identified elsewhere.
Finally, NLE is characterised by nonsuppurative leukoencephalitis with multifocal necrotic lesions affecting the white matter of the cerebrum, forebrain and thalamus29.
NME and NLE are differentiated from one another by anatomical lesion distribution. Malacic foci are observed in both forms, but for NLE are centred in the white matter of the cerebral hemispheres28,29.
Signalment
The usual example of MUO is the middle-aged neurological Bichon Frise or Maltese Terrier. While it is true that middle-aged, small breed dogs are over-represented in the literature, MUO can affect any dog of any age. In one study, it was revealed that 30 per cent of dogs diagnosed with GME specifically were large breed (>20kg)9. MUO also represents 25 per cent of all encephalopathies diagnosed in French bulldogs17, but it is unclear whether this is due to their recent surge in popularity or a true breed predisposition. Findings by Granger, Smith & Jeffery demonstrated that females are at a higher risk of developing the disease9.
The histological differentiation of MUO cannot be made ante-mortem and while the significance of this in a clinical setting is questionable, there are variations in signalment and presentation with each subtype of MUO. This may also be relevant for patient outcome as forebrain signs such as seizures, seen commonly in the necrotising form generally carry a worse prognosis2,6 . GME tends to affect middle-aged toy and terrier breeds5,9 whereas the necrotising form of the disease (NE) affects younger (<18 months) toy breeds with a great preponderance of pugs4,25 hence the name ‘Pug dog encephalitis’.
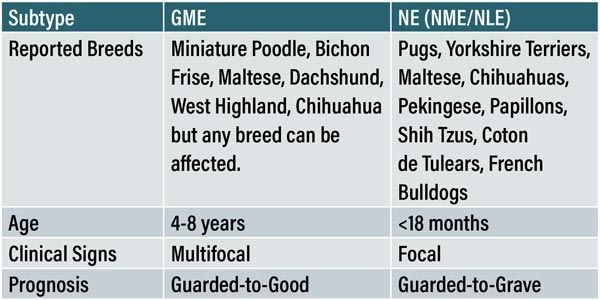
Table 1: Table adapted from Coates & Jeffery with reference to Talarico & Schatzberg, Cooper et al and Granger, Smith & Jeffery comparing the common presentations of both subtypes of MUO. Note that NME/NLE have been grouped together due to the lack of differentiation in the literature.
Clinical Presentation
One of the diagnostic challenges that MUO presents is its varied, non-specific presentation. Like any neurological condition, the clinical signs can provide clues to lesion location and while, hypothetically MUO can affect any region of the CNS, there are three main pathological distributions which have been described for GME specifically3,24: multifocal (disseminated), focal and ocular. Traditionally, multifocal GME is characterised by an acute, progressive onset of neurological signs. In contrast, focal lesions tend to display a more insidious onset like a space-occupying lesion. The third form, ocular GME can manifest as an acute onset of ocular dysfunction attributable to optic neuritis and anterior/posterior uveitis23. In general, clinical signs are variable and often ambiguous. Occasionally, animals with MUO may present with neck pain, tetra/paraparesis or ataxia.
It is important that a comprehensive clinical examination is performed on every animal as conditions such as Intervertebral disc herniation (IVDH) can present similarly to MUO and often require emergency surgical treatment.
The clinical history can suggest waxing and waning or episodic reports of symptoms which often display an inappropriate partial response to symptomatic treatment (for example: non-steroidal anti-inflammatories, antibiotics, or fluid therapy). Table 2 provides a brief description of some common clinical signs.
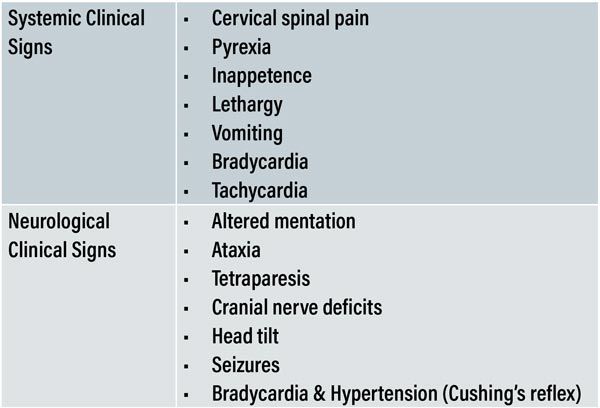
Table 2: Common systemic and neurological manifestations of MUO.
Diagnostic investigations
A definitive diagnosis of MUO can only be achieved via brain biopsy and subsequent histopathology, which while safe and accurate18, are restricted by financial constraints and limited accessibility. In the absence of brain biopsy or post-mortem confirmation, the disease is a diagnosis of exclusion using a combination of signalment and clinical presentation, CSF analysis results, MRI findings, and ruling out other plausible causes of systemic disease.
Granger et al (2010), reviewed 457 published cases to provide guidelines on patient selection in the absence of a brain biopsy and histopathology. The following inclusion criteria were established:
dogs older than 6 months of age;
multiple, single or diffuse intra-axial hyperintensities T2 weighted (T2W) MR images
Pleocytosis on CSF analysis with >50 per cent of monocytes/lymphocytes
Rule out infectious diseases commonly occurring in the specific geographic area.
Haematology and biochemistry are usually within normal limits or may reflect systemic inflammation such as neutrophilia, hyperglobulinaemia and hypoalbuminaemia; and interpretation of results should always consider patient signalment, clinical history and recent drug administration.
In the initial diagnostic stages blood results can often exclude metabolic or toxic causes such as hepatic encephalopathy, hypoglycaemia, hypocalcaemia, and hyper/hyponatraemia.
In addition to this, other ancillary tests such as Ammonia concentration, bile acid stimulation and ACTH stimulation can help further narrow down the list of differential diagnosis by assessing liver and adrenal gland function. Another notable diagnostic tool is C-reactive protein (CRP) which is an acute phase protein that is a highly sensitive indicator of systemic inflammation but with low specificity to the cause of inflammation1,2,16.
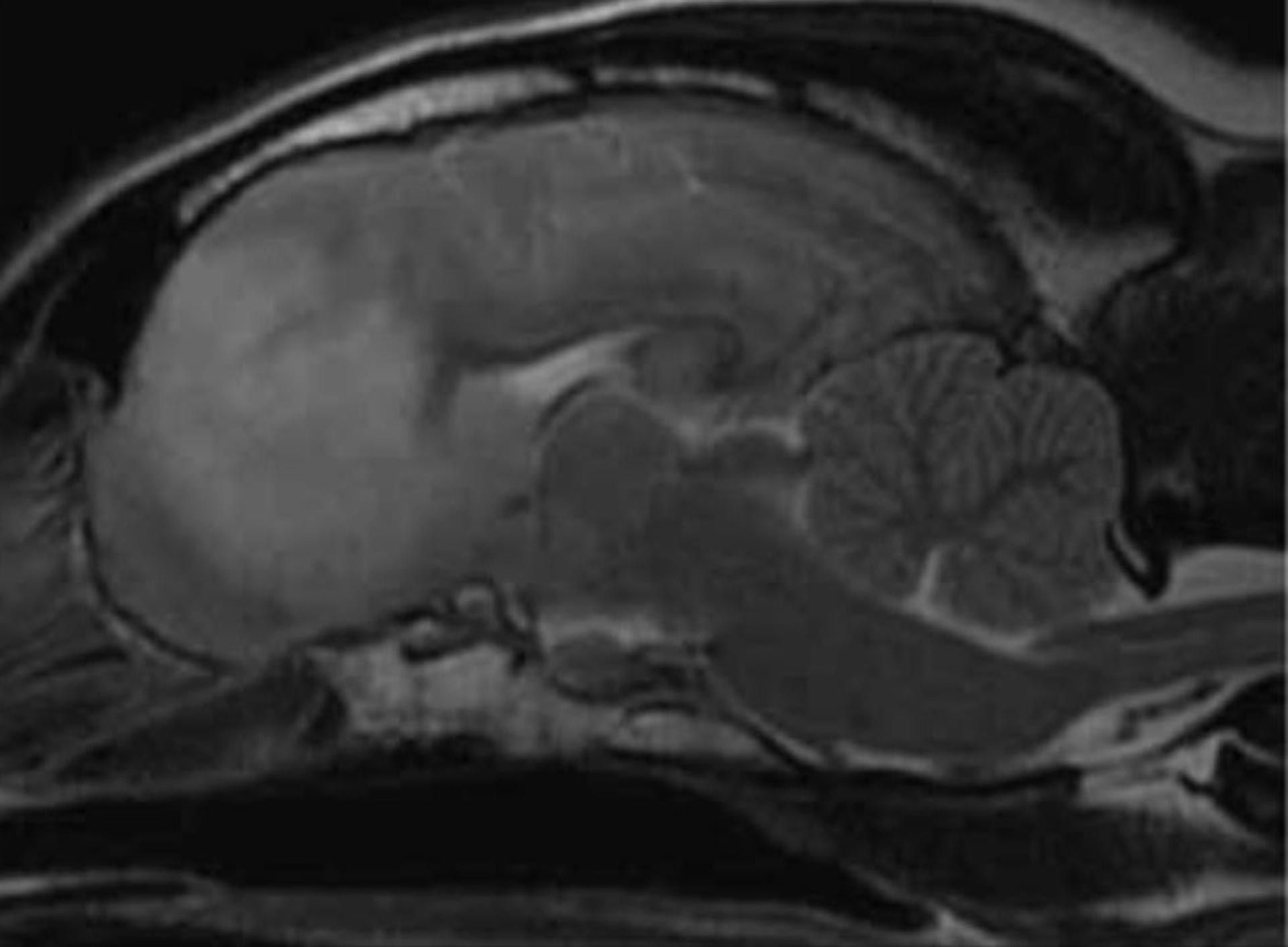
Figure 1: Sagittal T2W images of an eight-year-old female neutered Beagle diagnosed with MUO. Note the left olfactory and ventromedial aspect of the left frontal lobe hyperintensity affecting predominantly the white matter tracts on the T2W images. Image courtesy of Diagnostic Imaging services at UCD Veterinary Hospital.
MRI is the imaging modality of choice and has been reported to be 94.4 per cent sensitive and 95.5 per cent specific for detecting a brain abnormality5. However, it is important to note that not all patients with MUO will show changes on MRI and the sensitivity of imaging in identifying all inflammatory lesions suspected from the neurological examination remains quite low (<60 per cent)9. In contrast, Lamb et al (2005) determined that 76 per cent of cases with inflammatory CSF had concurrent MRI abnormalities13. There is no information on the use of MRI to differentiate between cases of GME, NME and NLE5.
It is important to note that a normal MRI result cannot eliminate MUO but could exclude other causes of neurological signs such as an intracranial mass for example. Most MUO cases will display hyperintense lesions on T2-weighted (T2W) images. T2-weighted images are useful for identifying abnormalities such as oedema and inflammation as they appear bright (hyperintense).
Another diagnostic criteria is the CSF analysis results including a cytology revealing pleocytosis. CSF can be collected from the cerebromedullary cistern or from the lumbar cistern15. CSF collection is contraindicated in patients that cannot be anaesthetised safely, have a suspected coagulopathy, and, most notably, for MUO patients with signs of increased intracranial pressure. CSF collection in a patient with increased intracranial pressure can be fatal due to brain herniation and compression of respiratory centres7. Fluid should be collected in an EDTA tube and slides made for prompt analysis as CSF fluid is susceptible to rapid degradation.
TNCC (total nucleated cell count) is fewer than five cells/uL in the dog and an increased TNCC is termed pleocytosis14. Typically, fluid cytology will reveal a mononuclear pleocytosis and increased total protein (> 30mg/dL) in the absence of an infectious cause. Generally, there is great variability between cell counts and some MUOs can have normal CSF results which can be linked to recent corticosteroid administration. Other less commonly described cell types are neutrophils and eosinophils with the later confirming eosinophilic meningoencephalomyelitis.
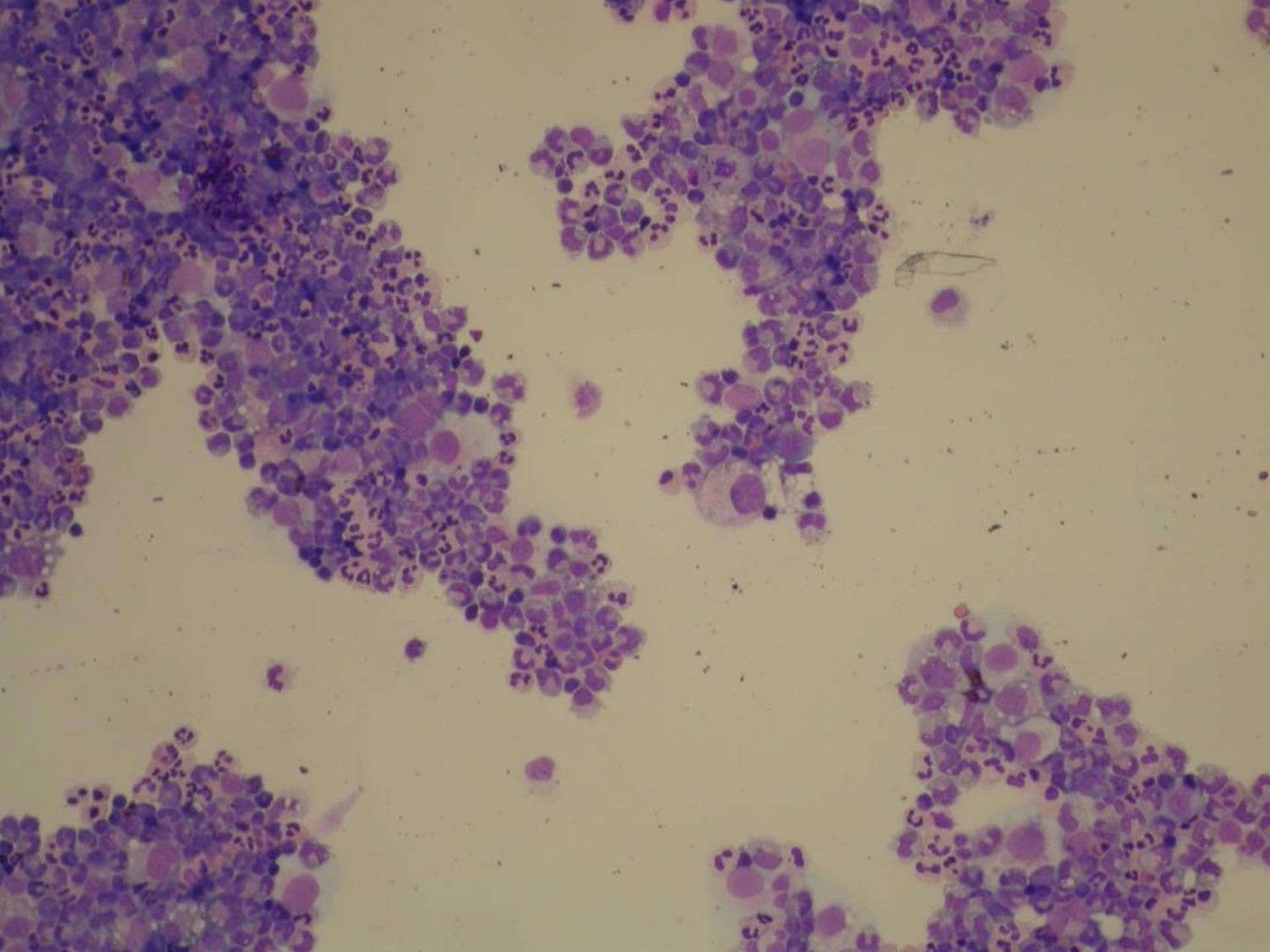
Figure 2: Cytology of a cytospin preparation of a cerebrospinal fuid sample (CSF) of a four-year-old female neutered Maltese dog diagnosed with MUO (100X amplification). Note the marked neutrophilic inflammation and occasional large mononuclear cells. Image courtesy of Dr Pedro Serra FRCP Dip.ACVP.
Treatment
Treatment of MUO remains to be one of the most controversial and challenging aspects of the condition due to the myriad of described aetiologies and unpredictable patient responses. In short, immunosuppressive therapy remains the mainstay of treatment. There is little evidence to support any specific regimen over another so individual clinical response should be used to guide treatment and prognostic decision-making. Often a standardised cytarabine-prednisolone protocol is used with the addition of secondary immunosuppressives such as a cyclosporin, mycophenolate and azathioprine as needed. Patients with MUO may also need anti-epileptic medication such as phenobarbital, levetiracetam and potassium bromide depending on their initial clinical presentation.
Prognosis and future research possibilities
Unfortunately, there is conflicting evidence on the prognostic indicators of this disease. It was thought originally that focal forebrain lesions were associated with a longer survival time19, however, more recent studies have not been able to replicate this theory6,15. In a study by Cornelis et al (2016) the presence of decreased mentation at time of presentation, seizures and increased neutrophil percentage in the CSF were all found to be significantly associated with death within seven days after diagnosis. However, these results may be biased due to the inclusion criteria used which favoured animals that may have been more severely affected. Furthermore, this study also emphasised a need to evaluate short-term prognostic factors as 26 percent of the dogs in the study died within one week of diagnosis.
Acknowledgments
The author would like to thank the entire Small Animal Internal Medicine team, notably Monica Augusto for her help with reviewing the article.
- Andersen-Ranberg E, Berendt M, Gredal H. Biomarkers of non-infectious inflammatory CNS diseases in dogs — Where are we now? Part I: Meningoencephalitis of unknown origin. Vet J. 2021 Jul;273:105678.
- Bathen-Noethen A, Carlson R, Menzel D, Mischke R, Tipold A. Concentrations of Acute-Phase Proteins in Dogs with Steroid Responsive Meningitis-Arteritis. J Vet Intern Med. 2008 Sep;22(5):1149–56.
- Braund KG. Granulomatous meningoencephalitis. J Am Vet Med Assoc. 1985;186:138–41.
- Cooper JJ, Schatzberg SJ, Vernau KM, Summers BA, Porter BF, Siso S, Young BD, Levine JM. Necrotizing meningoencephalitis in atypical dog breeds: a case series and literature review. J Vet Intern Med. 2014 Jan-Feb;28(1):198-203.
- Cornelis I, Van Ham L, Gielen I, De Decker S, Bhatti S.F.M. Clinical presentation, diagnostic findings, prognostic factors, treatment and outcome in dogs with meningoencephalomyelitis of unknown origin: A review. Vet J. 2019;244:37–44.
- Cornelis I, Volk HA, Van Ham L, De Decker S. Prognostic factors for 1-week survival in dogs diagnosed with meningoencephalitis of unknown aetiology. Vet J. 2016;214:91–95.
- Elias A, Brown C. Cerebellomedullary cerebrospinal fluid collection in the dog. Lab Anim. 2008;37:457-458.
- de la Fuente C, Monreal L, Cerón J, Pastor J, Viu J, Añor S. Fibrinolytic Activity in Cerebrospinal Fluid of Dogs with Different Neurological Disorders. Journal of Veterinary Internal Medicine. 2012 Aug 27;26(6):1365–73.
- Granger N, Smith PM, Jeffery ND. Clinical findings and treatment of non-infectious meningoencephalomyelitis in dogs: A systematic review of 457 published cases from 1962 to 2008. Vet J. 2010;184(3):290–297.
- Greer KA, Wong AK, Liu H, Famula TR, Pedersen NC, Ruhe A, Wallace M, Neff MW. Necrotizing meningoencephalitis of Pug Dogs associates with dog leukocyte antigen class II and resembles acute variant forms of multiple sclerosis. Tissue Antigens. 2010, Aug 76(2) 110-118.
- Jeffery N, Granger N. New insights into the treatment of meningoencephalomyelitis of unknown origin since 2009: A review of 671 cases. Front Vet Sci. 2023;10.
- Kipar A, Baumgärtner W, Vogl C, Gaedke K, Wellman M. Immunohistochemical Characterization of Inflammatory Cells in Brains of Dogs with Granulomatous Meningoencephalitis. Vet Pathol. 1998;35(1):43 52.
- Lamb CR, Croson PJ, Cappello R, Cherubini GB. Magnetic Resonance Imaging Findings in 25 dogs with Inflammatory Cerebrospinal Fluid. Veterinary Radiology Ultrasound. 2005 Jan;46(1):17–22.
- Levine GJ, Cook JR. Cerebrospinal Fluid and Central Nervous System Cytology. Cowell and Tyler’s Diagnostic Cytology and Hematology of the Dog and Cat. 2020:210–28
- Lowrie M, Smith PM, Garosi L. Meningoencephalitis of unknown origin: investigation of prognostic factors and outcome using a standard treatment protocol. Vet Rec. 2013;172(20):527–527.
- Lowrie M, Penderis J, Eckersall PD, McLaughlin M, Mellor D, Anderson TJ. The role of acute phase proteins in diagnosis and management of steroid-responsive meningitis arteritis in dogs. Vet J. 2009 Oct;182(1):125–30.
- Mayousse V, Desquilbet L, Jeandel A, Blot S. Prevalence of neurological disorders in French bulldogs: a retrospective study of 343 cases (2002–2016). BMC Vet Res. 2017 Jul 5;13(1).
- P. Moissonnier, Blot, S., Devauchelle, P., Delisle, F., Frédéric Beuvon, Boulha L, Colle, M.-A. and Lefrancois, T. (2002). Stereotactic CT‐guided brain biopsy in the dog. 43(3), pp.115–123. doi:https://doi.org/10.1111/j.1748-5827.2002.tb00041.x.
- Muñana KR, Luttgen PJ. Prognostic factors for dogs with granulomatous meningoencephalomyelitis: 42 cases (1982-1996). 212(12):1902–6.
- Nessler JN, Oevermann A, Schawacht M, Gerhauser I, Spitzbarth I, Bittermann S, Steffen F, Schmidt MJ, Tipold A. Concomitant necrotizing encephalitis and granulomatous meningoencephalitis in four toy breed dogs. Front Vet Sci. 2022;9.
- O’Neill EJ, Merrett D, Jones B. Granulomatous meningoencephalomyelitis in dogs: A review. Ir Vet J. 2005;58(2):86.
- Pedersen N, Liu H, Millon L, Greer K. Dog Leukocyte Antigen Class II–Associated Genetic Risk Testing for Immune Disorders of Dogs: Simplified Approaches Using Pug Dog Necrotizing Meningoencephalitis as a Model. J Vet Diagn Invest. 2011;23(1):68–76.
- Smith R. A case of ocular granulomatous meningoencephalitis in a German Shepherd Dog presenting as bilateral uveitis. Aust Vet Pract. 1995;25:76-8.
- Sorjonen DC. Clinical and histopathological features of granulomatous meningoencephalomyelitis in dogs. J Am Anim Hosp Assoc. 1990;26:141–7.
- Talarico LR, Schatzberg SJ. Idiopathic granulomatous and necrotising inflammatory disorders of the canine central nervous system: a review and future perspectives. J Small Anim Pract. 2010 Mar;51(3):138-49.
- Thomas WB. Inflammatory diseases of the central nervous system in dogs. Clin Tech Small Anim Pract. 1998 Aug;13(3):167–78.
- Uchida K, Hasegawa T, Ikeda M, Yamaguchi R, Tateyama S. Detection of an Autoantibody from Pug Dogs with Necrotizing Encephalitis (Pug Dog Encephalitis). Vet Pathol. 1999;36(4):301–307.
- Uchida K, Park E, Tsuboi M, Chambers JK, Nakayama H. Pathological and immunological features of canine necrotising meningoencephalitis and granulomatous meningoencephalitis. Vet J (London, England: 1997). 2016;213:72–77.
- Conference 12 - 2017 Case: 4 20171213 [Internet]. www.askjpc.org. [cited 2023 Oct 12].
Which of the following is NOT a subtype of Meningoencephalomyelitis of Unknown Origin?
A. Granulomatous Meningoencephalomyelitis (GME)
B. Necrotising Meningoencephalomyelitis (NME)
C. Steroid Responsive Meningitis (SRM)
D. Necrotising Leukoencephalitis (NLE)
2. What is the primary diagnostic modality of choice for detecting brain abnormalities in dogs with Meningoencephalomyelitis of Unknown Origin (MUO)
A. Blood tests
B. Radiographs
C. CT (Computed Tomography)
D. MRI
3. What type of cells are predominantly found within Granulomatous Meningoencephalomyelitis (GME) lesions, suggesting a T-cell mediated immune response?
A. Neutrophils
B. B cells
C. Eosinophils
D. Mononuclear cells, including lymphocytes and macrophages
4. In terms of prognosis which subtype of Meningoencephalomyelitis of Unknown Origin (MUO) tends to have a worse prognosis when clinical signs include seizures?
A. Granulomatous Meningoencephalomyelitis (GME)
B. Necrotising Meningoencephalomyeitis (NME)
C. Necrotising Leukoencephalitis (NLE)
D. All subtypes have the same prognosis when seizures are present.
5. What is the mainstay of treatment for dogs with MUO?
A. Surgical intervention
B. Antibiotics
C. Immunosuppressive therapy
D. Anti-epileptic medication
ANSWERS:1C; 2D; 3D; 4D; 5C.















