A palliative treatment modality for appendicular osteosarcoma in a dog
Pietro Sabbatini MVB, currently undertaking a small animal surgical internship at MyVet Referrals Lucan under the guidance of Ciarán Jones MVB MSDipl ECVSDipl ACVS, describes the surgical treatment of a dog with proximal humeral osteosarcoma using a new injectable, self-setting bone substitute* as a new option in the palliative care of this disease
Background
Osteosarcoma (OSA) is a primary, malignant, monostotic, mesenchymal tumour of bone cells, accounting for 75 per cent to 85 per cent of primary bone neoplasia in dogs.1,2 It mostly arises in the medullary cavity, it is locally aggressive, and it causes destruction of bone and surrounding soft tissue.2,3 Osteosarcomas rarely invade adjacent bones or cross a joint.3 Early ovariohysterectomy, castration and chronic inflammatory processes have been associated with an increased risk of OSA development.1,2,4 OSAs seem to have a biphasic peak of age occurrence: large and giant-breed dogs are over-represented with a median age of seven years, but a smaller, earlier peak incidence was also identified in animals between 18 and 24 months of age.1,2,3,4 A breed-predisposition has also been demonstrated: Greyhound, Rottweiler, Great Dane, St Bernard, Irish Setter, Dobermann, German Shepherd Dog and Golden Retriever are at an increased risk of developing this cancer.2,4
Overall, appendicular skeletal osteosarcoma accounts for 75 per cent of all OSAs, with forelimbs being affected twice as often as hindlimbs.1,3 Distal radial metaphysis and proximal humerus are the most common locations3,4, followed by distal femur and proximal tibia (i.e., “towards the knee and away from the elbows”). OSAs are thought to have a predilection for the metaphyseal region of bones due to the high metabolic activity and excellent blood supply characteristic of this area.3 Lameness and localised limb swelling are key features of appendicular OSA; this usually presents as a chronic and progressive process, but onset can also be acute and severe when pathologic fractures are involved.1 Metastases are extremely common with appendicular OSAs and usually occur in the lungs via the haematogenous route4; although there may not be initial radiographic evidence of metastatic disease, 90 per cent of patients die or are euthanised because of complications secondary to pulmonary metastases within the first year of diagnosis. On the other hand, although axial skeletal OSAs are thought to have a lower metastatic potential, they still carry a poor prognosis due to the difficulties encountered in controlling the spread of the primary tumour.5 Overall, the presence of detectable metastases at the time of diagnosis remains the single most important prognostic indicator.4 Other negative prognostic factors include: patient’s age; tumour’s histologic grade and location; lymph nodes involvement; an elevated blood alkaline phosphatase.2,4 Although the presumptive diagnosis for an aggressive, monostotic bone lesion should always be osteosarcoma, other possible differential diagnoses include bacterial and fungal osteomyelitis, other primary bone tumours, metastatic bone disease, subchondral bone cysts and inflammatory periosteal responses.
Diagnostic procedures
Common diagnostic imaging procedures include radiographs of the affected bone and a three-view radiographic survey of the thorax. An active ‘sun-burst’ periosteal reaction – i.e., new tumour bone forming in a palisading pattern, radiating from the axis of the cortex – is the characteristic radiographic sign for OSA. This usually presents as a single, isolated cortico-trabecular bone lesion: such lesions can be predominantly lytic, proliferative or it can follow a mixed pattern.3
Contrast-enhanced computed tomography (CT) has a higher sensitivity than radiography in detecting pulmonary micro-metastases as the first can reliably identify nodules as small as 1mm in diameter6, while the latter cannot detect nodules smaller than 6-8mm.2 Magnetic resonance imaging (MRI) and bone scintigraphy can also give a detailed screening of the extent of the disease, but are less commonly performed due to their elevated cost and limited availability.2,4
Tradtional treatment options
Treatment options for OSA are either aimed at slowing down cancer progression or can simply have a palliative intent; a further subdivision can be made between medical and surgical therapies.
To date, the gold standard of treatment is amputation followed by earliest possible administration of adjuvant chemotherapy (cisplatin, carboplatin, doxorubricin, or combinations), yielding a mean survival time (MST) of 11-14 months from the time of diagnosis.2,4 Limb-sparing surgical techniques (LSST) can also be valuable alternatives when amputation is not an option, such as with dogs having pre-existing orthopaedic or neurologic deficits, in cases of severe obesity or when owners simply refuse amputation7. However, these techniques usually require specific surgical training and expertise, and cost and owner commitment can be significant. The standard LSST consists of marginal en-bloc resection of the tumour bone, replacement by bone allograft to reconstruct the bony column and plating of the defect, with or without arthrodesis of the adjacent joint.4,7
When compared to amputation and chemotherapy, LSSTs were noted to carry a higher risk of perioperative complications such as infections, local tumour recurrence (20-30 per cent), fractures and implant failure.4,8 A combination of LSST and chemotherapy (cisplatin, carboplatin, doxorubicin or combinations) yielded an MST of 12-16 months and mostly succeeded in providing a functional, pain-free limb.4 Interestingly, the occurrence of chronic surgical-site infections after limb-sparing surgeries has also been shown to increase the MST2,4,7; this is thought to be a consequence of the activation of immune effector cells targeting neoplastic cells and inhibiting tumour metastatic growth.4,9 Common palliative treatment options include limb-sparing techniques – such as long-term administration of analgesics and radiation therapy (both yielding an MST of two to four months4), or amputation alone as a means to remove the source of pain (MST of three to six months4).
Limb-sparing palliative therapies were shown to potentially increase the incidence of pathologic fractures due to an increase in weight-bearing in the diseased limb as a consequence of reduced pain, or – limited to radiation therapy – due to a decrease in the intrinsic strength of bone.10,11 On the other hand, a study has shown how in situ treatment of OSA can be beneficial, as antigenic proteins released from OSAs are believed to trigger a systemic immune response targeting neoplastic cells and potentially reducing local tumour recurrence and delaying the occurrence of metastatic disease.11
In this regard, a new palliative limb-sparing technique was recently made available and consists in performing a percutaneous injection of a synthetic, self-hardening calcium-phosphate bone substitute directly into the lesion to fill the void caused by the bone tumour – i.e., a “cementoplasty”. According to the clinical trials available to date, this treatment is capable of increasing the mechanical strength of the weakened tumour bone, therefore reducing pain and risks of developing pathological fractures.12 Percutaneous cementoplasty using a different bone substitute (PMMA) was previously attempted as part of multimodal management of canine OSA, but treatment outcomes were not as encouraging due to the high rate of post-operative complications encountered.13
Case presentation and choice treatment modality
A 12-year-old, female entire Collie presented with a four-week history of progressive left forelimb lameness which only partially and transiently responded to rest and anti-inflammatories. On clinical examination, a severe, almost non-weight-bearing left forelimb lameness was confirmed at a walk, pain was elicited on palpation of the left proximal humerus, and mild-to-moderate muscle atrophy was present in the left shoulder region. No significant abnormalities were detected on haematology and biochemistry panels. A radiographic study of the left forelimb showed the presence of a single cortico-trabecular bone lesion in the proximal humerus, characterised by a mixed lytic and proliferative pattern and a ‘sun-burst’ periosteal reaction (Figure 1). Given the signalment, clinical signs, radiographic characteristics and location of the lesion, a pre-emptive diagnosis of appendicular osteosarcoma was made. Survey radiographs of the thorax showed no evidence of detectable metastatic disease at the time of diagnosis.
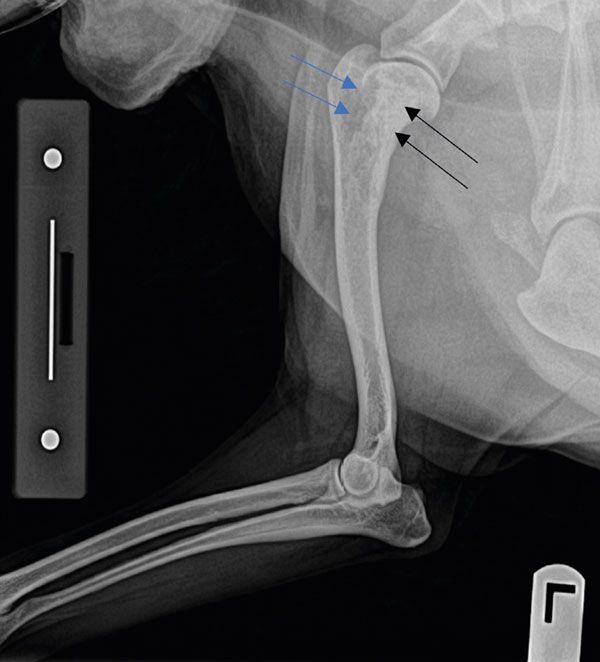
Figure 1: A focal, ill-defined, poorly marginated cortico-medullary bony lesion and a “sunburst” periosteal reaction were identified in the left proximal humerus. This lesion shows a mixed lytic (blue arrows) and proliferative (black arrows) pattern.
Given the owner’s reluctance to opt for an amputation, palliative and limb-salvage techniques were offered. In a study conducted on 17 client-owned dogs, limb salvage surgery for OSA of the proximal humerus was found to give unacceptable outcomes and high post-operative complication rates, with ultimate limb function being poor in 59 per cent of the dogs.8 This anatomical region was also shown to be one of the most common locations for the occurrence of OSA-related pathological fractures, likely because structural bone damage at this site may go unnoticed due to the significant amount of soft tissue coverage compared with other commonly affected areas.14
For these reasons, while traditional surgical limb sparing techniques were not considered to be valuable options for this patient, percutaneous cementoplasty was found to potentially offer several advantages, especially when taking this patient’s old age into account as an additional risk factor:
being a minimally invasive, low-traumatic technique, it would guarantee a more rapid post-operative recovery;
the patient would face a reduced risk associated with general anaesthesia given the short procedure time;
given the expected pain reduction after the procedure, an overall decrease in long-term administration of analgesics would reduce the risk of systemic side-effects associated with these;
this treatment can reduce the likelihood of developing pathological fractures – this is a key point, given the propensity for them to occur in this given anatomical area;
due to the large amount of soft tissue cover in this region, the sterility of the procedure and the absence of implants, this patient would also be at low risk of developing surgical site infections;
from a financial perspective, this procedure is more accessible than amputation.
SURGICAL TREATMENT: PERCUTANEOUS CEMENTOPLASTY
A cranio-lateral approach to the left proximal humerus was performed; an approximately 4cm skin incision was made, and blunt soft tissue dissection allowed exposure of the lateral aspect of the proximal humeral cortex. A 23Gx1 ¼" hypodermic needle was placed in the area of the bone lesion (Figure 2) and x-rays were taken to confirm correct positioning of the needle (Figure 3).
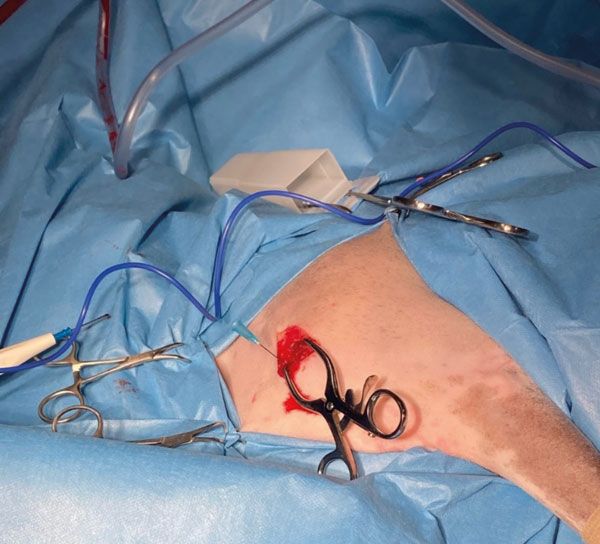
Figure 2.
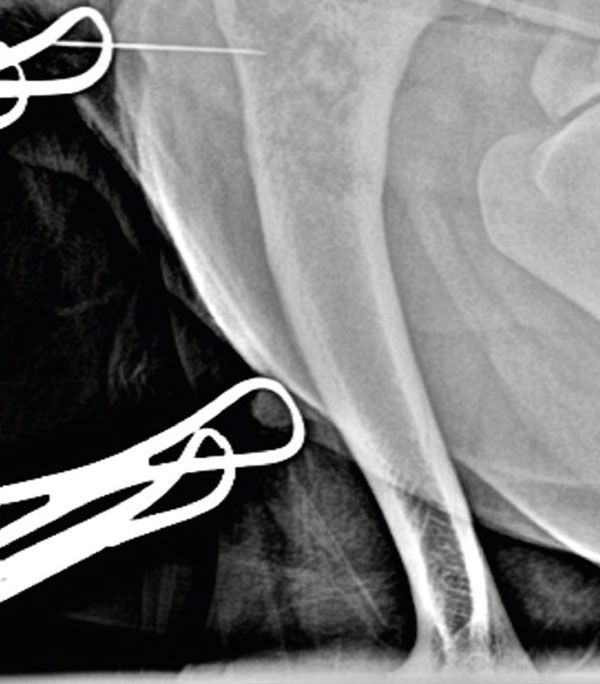
Figure 3.
Using the needle as a landmark, a bone tunnel was opened into the defect with a 1.1mm Kirschner wire (Figure 4): the hole was progressively enlarged using 3.5mm and then 4.5mm drill-bits. Such a tunnel should always be drilled starting from healthy bone and aiming into the lesion, to minimise the risks of fracturing the bone weakened by the tumour and seeding neoplastic cells.
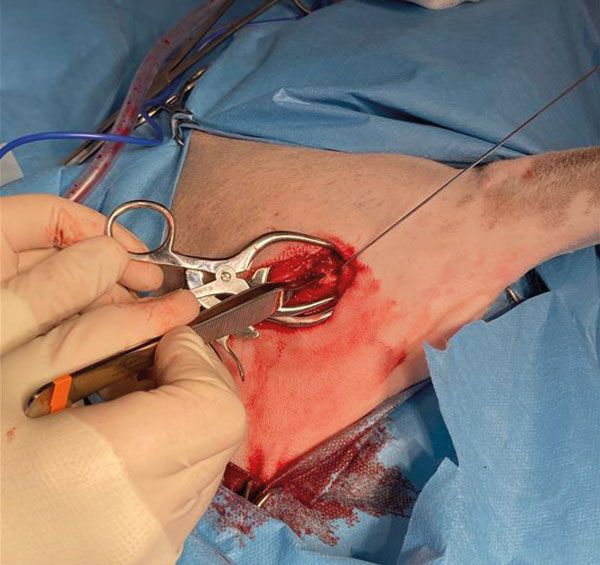
Figure 4.
The lesion was then suctioned to remove as many tumour cells as possible and to make room for the injectable bone substitute. Approximately 10ml of the product were injected into the lesion (Figure 5): thanks to the product’s radiopacity, this procedure could be carried out under radiographic guidance to ensure adequate filling of the bone defect was achieved.
A second bone tunnel was created more proximally to the first entry point, to allow further administration of the cement.
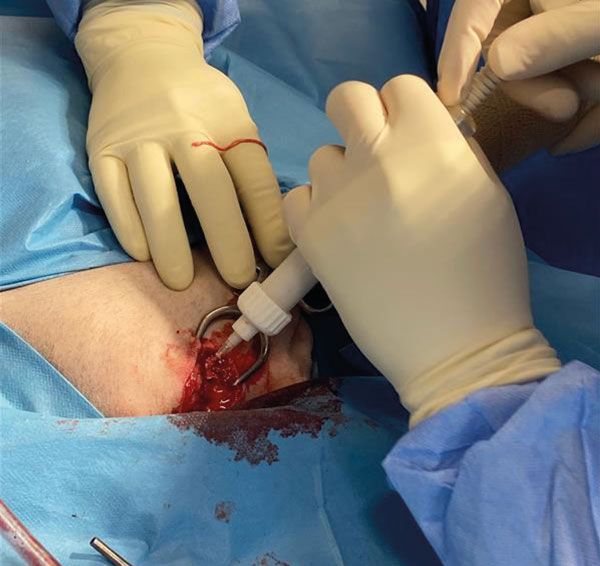
Figure 5.
The surgery site was then thoroughly lavaged, the wound was routinely closed, and post-operative x-rays were taken to assess the degree of filling of the lesion (Figure 6). Contact between the bone substitute and intact surrounding cortical bone is recommended for a positive outcome, along with maximal filling of the defect. Calcium-phosphate bone substitute is expected to achieve a mechanical strength comparable to cancellous bone within the first 24 hours post-operatively.12 When available, fluoroscopy is a valuable option for real-time monitoring of percutaneous injections.

Figure 6.
POST-OPERATIVE FOLLOW-UP
The patient was discharged from the hospital the following day and post-operative pain was managed with oral administration of paracetamol and metacam. A course of antibiotics was also initiated to further reduce the risk of surgical site infection, given the significant tissue damage caused by the tumour. At the two-week post-operative check, the patient showed significantly increased comfort levels, was fully weight-bearing and only a mild, intermittent left forelimb lameness was noticed at a walk. The owner also perceived that the dog was remarkably more comfortable and had an improved limb function compared to before surgery. At the six-week post-operative check, the patient showed a moderate left forelimb lameness and was started back on oral metacam. A radiographic study of the left forelimb and chest were also performed at that time, showing no further bone lysis and no thoracic metastases. The patient was humanely euthanised two months after surgery, secondary to a sudden deterioration of its quality of life.
CONCLUSION
Percutaneous cementoplasty using calcium-phosphate bone substitute as a palliative treatment for osteosarcoma showed promising results: the outcomes of this study were in line with the currently available data, which showed a reduction in lameness and an improved limb function in 67 per cent and 50 per cent of treated dogs at one- and two-months post-surgery respectively.12
Overall, survival time was found to be similar to that of dogs treated with amputation alone, with the additional benefit of maintaining four functional legs in an elderly patient.
Survival time was also certainly in line with the MST associated with other limb-sparing techniques, such as administration of analgesics and radiation therapy.
Given the minimally invasive, low-trauma, low-risk and quick nature of this procedure, percutaneous cementoplasty could become a safe alternative to the standard palliative treatment options; this is particularly important in countries like Ireland where radiation therapy is not yet available, and the number of palliative treatment modalities is still limited. Percutaneous cementoplasty could also become part of multimodal treatment modalities: as in this case, it could be combined with analgesics to maximise the patient’s comfort levels while minimising risks associated with long-term administration of pain medication (such as systemic side-effects or pathologic fractures). It could also be combined with chemotherapy and with several advanced limb-sparing surgical techniques (such as microwave thermal ablation), where it would aim at increasing bone strength by acting as a bone stabiliser.15
In this case, chemotherapy was recommended and would have likely further prolonged survival time but was declined by the owner.
To date, only one prospective, non-controlled clinical trial on 12 client-owned dogs is available on this technique. Therefore, prospective controlled studies with bigger group sizes should be carried out to further investigate this treatment modality. To the authors’ knowledge, this is the first time this technique was performed to treat appendicular osteosarcoma in a dog in the Republic of Ireland.
* BIOCERA-VET®OSA RTU (TheraVet, Gosselies, Belgium).
Boston S. Musculoskeletal Neoplasia and Limb-Sparing Surgery. In: Johnston SA, Tobias KM, editors. Veterinary surgery: Small animal. St. Louis: Elsevier Saunders; 2012. p. 1159.
2. Schulz KS, Hayashi K, Fossum TW. Other Diseases of Bones and Joints. In: Fossum TW, editor. Small animal surgery. Philadelphia: Elsevier; 2019. p.1299-1308.
3. McAllister H, Tobin E. Long bones – mature. In: Kirberger RM, McEvoy FJ, editors. BSAVA manual of Canine and Feline Musculoskeletal Imaging. 2nd ed. Gloucester: British Small Animal Veterinary Association; 2016. p. 122-126.
4. Dernell WS. Tumours of the skeletal system. In: Dobson JM, Lascelles BD, editors. BSAVA Manual of Canine and Feline Oncology. 3rd ed. Gloucester: British Small Animal Veterinary Association; 2011. p. 159-173.
5. Heyman SJ, Diefenderfer DL, Goldschmidt MH, Newton CD. Canine axial skeletal osteosarcoma a retrospective study of 116 cases (1986 to 1989). Veterinary Surgery. 1992;21(4):304–310. Available at: https://doi.org/10.1111/j.1532-950x.1992.tb00069.x.
6. Nemanic S, London CA, Wisner ER. Comparison of thoracic radiographs and single breath-hold helical CT for detection of pulmonary nodules in dogs with metastatic neoplasia. Journal of Veterinary Internal Medicine. 2006;20(3):508–515. Available at: https://doi.org/10.1111/j.1939-1676.2006.tb02889.x.
7. Liptak JM, Dernell WS, Ehrhart N, Lafferty MH, Monteith GJ, Withrow SJ. Cortical allograft and endoprosthesis for limb-sparing surgery in dogs with distal radial osteosarcoma: A prospective clinical comparison of two different limb-sparing techniques. Veterinary Surgery. 2006;35(6):518–533. Available at: https://doi.org/10.1111/j.1532-950x.2006.00185.x.
8. Kuntz CA, Asselin TL, Dernell WS, Powers BE, Straw RC, Withrow SJ. Limb salvage surgery for osteosarcoma of the proximal humerus: Outcome in 17 dogs. Veterinary Surgery. 1998;27(5):417–422. Available at: https://doi.org/10.1111/j.1532-950x.1998.tb00150.x.
9. Hans EC, Pinard C, van Nimwegen SA, Kirpensteijn J, Singh A, MacEachern S, et al. Effect of surgical site infection on survival after limb amputation in the curative‐intent treatment of canine appendicular osteosarcoma: A veterinary society of surgical oncology retrospective study. Veterinary Surgery. 2018;47(8). Available at: https://doi.org/10.1111/vsu.13105.
10. Covey JL, Farese JP, Bacon NJ, Schallberger SP, Amsellem P, Cavanaugh RP, et al. Stereotactic Radiosurgery and fracture fixation in 6 dogs with appendicular osteosarcoma. Veterinary Surgery. 2014;43(2):174–181. Available at: https://doi.org/10.1111/j.1532-950x.2014.12082.x.
11. Steffey MA, Garcia TC, Daniel L, Zwingenberger AL, Stover SM. Mechanical properties of canine osteosarcoma-affected antebrachia. Veterinary Surgery. 2017;46(4):539–548. Available at: https://doi.org/10.1111/vsu.12628.
12. Abstracts from the ESVONC Annual Congress, May 26–28, 2022, Siracusa, Sicily: Villamonte Chevalier A, Molle C, Carabalona J, Klajer A, Letesson J, Ragetly G, et al. Percutaneous cementoplasty as a palliative treatment for dogs with osteosarcoma using a new self-setting bone substitute. Vet Comp Oncol. 2022;20(S2):3-17. Available at: https://doi.org/10.1111/vco.12850.
13. Böttcher P, Krastel D, Hierholzer J, Westphalen K, Florian S, Hildebrandt G, et al. Percutaneous cementoplasty in the palliative, multimodal treatment of primary bone tumours of the distal aspect of the radius in four dogs. Veterinary Surgery. 2009;38(7):888–901. Available at: https://doi.org/10.1111/j.1532-950x.2009.00596.x.
14. Bhandal J, Boston SE. Pathologic fracture in dogs with suspected or confirmed osteosarcoma. Veterinary Surgery. 2011;40(4):423–430. Available at: https://doi.org/10.1111/j.1532-950x.2011.00811.x.
15. Abstracts from the ESVONC Annual Congress, May 26–28, 2022, Siracusa, Sicily: Sayag D, Jacques D, Thierry F, Gousset G, Castell Y, Aumann M, et al. CT-guided microwave thermal ablation and cementoplasty as a part of the management of appendicular osteosarcoma in a dog. Vet Comp Oncol. 2022;20(S2):3-17. Available at: https://doi.org/10.1111/vco.12850.
1. THE MOST COMMON LOCATIONS FOR APPENDICULAR OSTEOSARCOMA ARE:
A. Proximal femur and distal tibia
B. Distal radial metaphysis and proximal humerus
C. Proximal radius and distal humerus
D. Mid-diaphyseal region of humerus and tibia
2. THE GOLD STANDARD OF TREATMENT FOR APPENDICULAR OSTEOSARCOMA IS:
A. Amputation alone
B. Radiotherapy
C. Anti-inflammatories and chemotherapy
D. Amputation followed by adjuvant chemotherapy
3. TRUE OR FALSE: CT IS THE MOST VALUABLE DIAGNOSTIC IMAGING MODALITY WHEN SCREENING FOR PULMONARY METASTASES
A. True: it can detect pulmonary nodules up to 6-8mm in diameter
B. False: MRI has a higher sensitivity and is more accessible than CT
C. False: radiography has a higher sensitivity and can detect pulmonary nodules down to 6-8mm in diameter
D. True: CT is more accessible than MRI and can detect pulmonary nodules down to 1mm in diameter
4. A KEY BENEFIT BROUGHT BY PERCUTANEOUS CEMENTOPLASTY IS:
A. An increase in post-operative bone strength and a consequent decrease in risk of developing pathologic fractures
B. A reduced incidence of metastatic spread of the primary bone tumour
C. A reduced risk of local spread of the tumour
D. An increase in MST due to its capability of slowing down cancer progression
5. 90 PER CENT OF PATIENTS DIE OR ARE EUTHANISED WITHIN THE FIRST YEAR OF APPENDICULAR OSA DIAGNOSIS:
A. Due to complications secondary to pulmonary metastases
B. Due to the local spread of the tumour
C. Due to complications secondary to metastases to liver and spleen
D. Due to the low comfort levels and pain caused by the tumour
ANSWERS: 1B; 2D; 3D; 4A; 5A.









