Surgical management of HO in a dog
Pietro Sabbatini MVB, working as a small animal surgical intern with the specialist surgeon Ciarán Jones MVB, MS, Dipl. ECVS, Dipl. ACVS at MyVet Referrals Lucan, discusses the clinical presentation, diagnosis and surgical management of a case of hypertrophic osteopathy (HO) in a dog
Hypertrophic osteopathy (HO) is a pathology characterised by deposition of new periosteal bone at the distal extremities and long bones, secondary to the presence of a large, chronic, space-occupying lesion in the thoracic and/or abdominal cavity. It usually affects middle-aged to geriatric dogs (cats rarely develop this condition) and it is often associated with both lameness and a firm, non-pitting, irregular soft tissue swelling at the affected sites. Lameness onset is often insidious, it tends to be more pronounced at an earlier stage and can range from mild to non-weight bearing.11 Clinical signs related to the location of the primary mass can also be part of the clinical picture, although lameness tends to manifest first. Other clinical signs include hyperthermia, lethargy and weight loss. The most relevant differential diagnoses include osteomyelitis, neoplasia, osteoarthritis, hypertrophic osteodystrophy, multiple cartilaginous exostosis, panosteitis and- limited to feline patients- hypervitaminosis A.11
The periosteal reaction is deemed to have neurovascular implications: irritation of the vagus, intercostal or other afferent nerves caused by the primary disease is thought to be capable of eliciting a nervous reflex which produces a rapid increase in peripheral blood flow. This, in turn, leads to proliferation of connective tissue and periosteal bone.13 This mechanism has been at least partially confirmed as clinical signs of HO usually reduce after surgical removal of the primary mass or bilateral cervical vagotomy11 ; an improvement in patients’ condition can be seen as early as one week post-operatively. Other reported aetiological theories propose toxins circulating from the mass to be the inciting cause of the periosteal reaction5, or else the involvement of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) in HO initiation and progression.14
Neoplastic primary or metastatic lung conditions are by far the most common pathologies associated with HO, with approximately 15 per cent of affected dogs eventually developing Marie’s disease. 4 Several other neoplastic and non-neoplastic diseases have been related to HO, such as intra-thoracic abscessation11, nephroblastoma13, prostatic carcinoma1, oesophageal neoplasia secondary to spirocercosis11,12 pyometra10 and foreign bodies6, to mention a few.
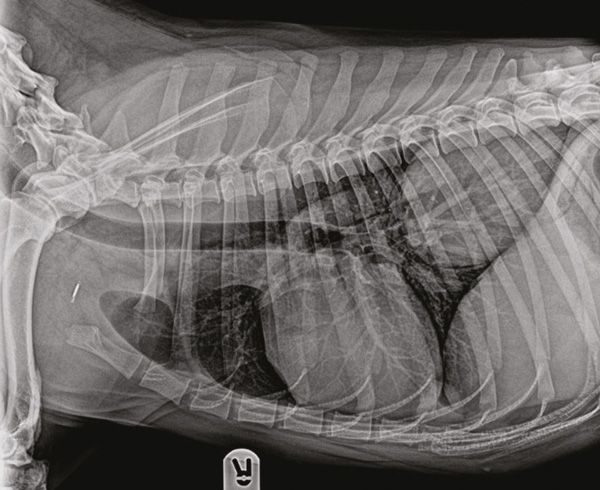
Figure 1: Right lateral view of the thorax. A large mass can be noted in the right caudal lung lobe.
Ideally, a three-view thoracic and two-view abdominal radiologic study should always be part of the diagnostic database when screening for this disease.11 Radiographic bony changes (which may not be immediately evident in the early stages of disease) consist of a polyostotic, bilaterally symmetrical periosteal proliferation at the diaphysis of distal long bones, with subsequent progression to more proximal bones.11 This proliferation is often described as ‘palisading’, as periosteum deposition occurs at right angles to the long axis of the bone. It usually starts on the distal abaxial surface of bones10 and it appears to be ‘scalloped'. It is important to note that this condition always spares the joint spaces.3 Over time, the new bone deposed becomes rounder and smoother and it begins to be laid down in parallel layers with the bone cortex. Occasionally, new bone can get deposed in the soft tissues and be completely isolated from the bone cortex.7
When surgery is not an option, medical management of the underlying primary condition is advised, along with analgesia and supportive care.11
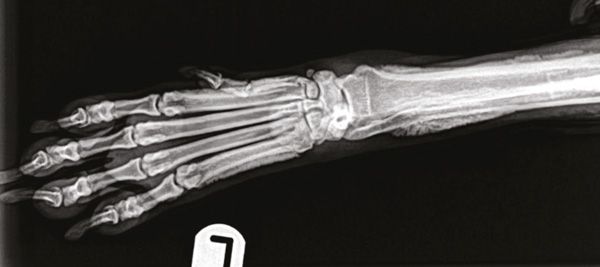
Figure 3: Dorsopalmar view of the left distal forelimb. The new periosteal bone is sharply marginated with distinct edges: its shape is irregular, almost palisading. As a result of the new bone deposition, there also appears to be increased opacity in the medullary cavity of these bones.
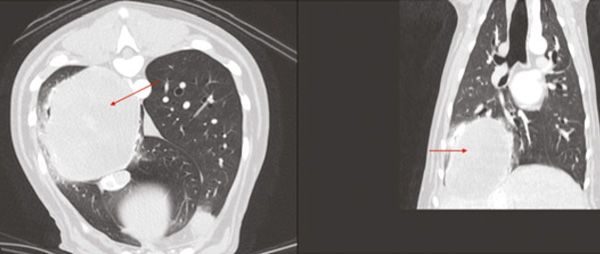
Figure 4: Pulmonary mass (arrows) noted on Computed Tomography.
Case presentation
A four-year-old, female, neutered Shepherd cross presented with a six-week history of lethargy, increased respiratory rate and licking at the paws and a three-week history of intermittent forelimb lameness and discomfort. Additionally, an approximately two-month history of bilateral ocular discharge and scleral hyperaemia were reported.
Blood test results
Despite mainly showing only mild abnormalities, blood results disclosed characteristic changes associated with HO:
A subtle neutrophilia, which can be associated with stress or inflammation either surrounding new bone formation or secondary to the primary disease process.13
A marked decrease in platelet counts. In a study conducted in human patients affected by HO, platelet abnormalities have been postulated to be caused by conditions altering the normal pulmonary circulation, such as chronic lung pathologies. Such alteration would impair the normal platelet fragmentation process, resulting in macroplatelets that are known to affect the platelet count.14
A mild decrease in serum Albumin (Alb), which can be associated with the chronic, ongoing inflammatory process as this is a negative acute phase protein.
A mild increase in Alkaline Phosphatase (ALP): this can be caused by the new periosteal deposition, as the ALP bone isoenzyme is a widely accepted marker of new bone formation and early osteoblast activity.13
Diagnostic imaging
Radiographs highlighted a lung mass, along with a polyostotic, ‘palisading’ periosteal proliferation in all four distal limbs.
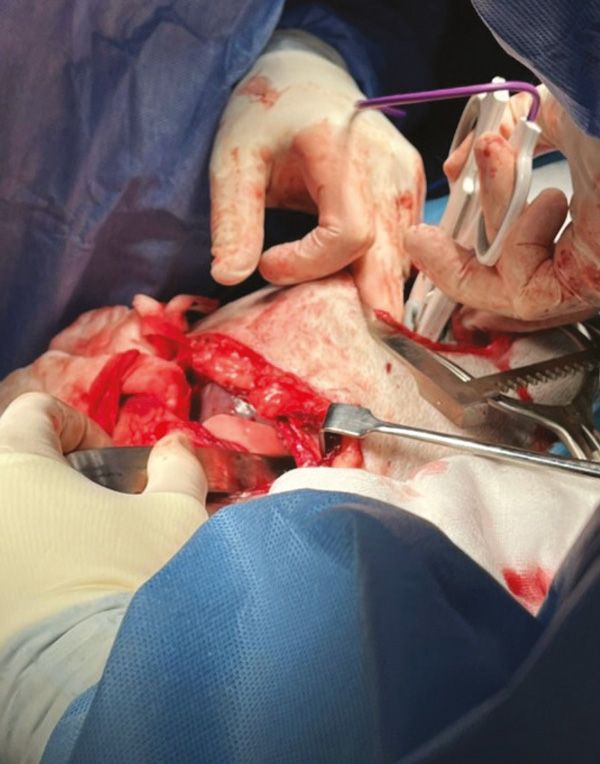
Figure 5: Breakdown of adhesions with Ligasure device.
CT of the thorax and abdomen revealed a large (8.4 x 8.1 x 6.4 cm) pulmonary mass located in the right caudal lung lobe. No metastases were detected.
Surgical treatment
An exploratory thoracotomy and right caudal lung lobectomy were performed to surgically excise the mass. A standard lateral intercostal thoracotomy was performed at the fifth right intercostal space (ICS). A self-retaining, Finochietto rib retractor was used to provide enhanced access to the chest cavity. The right caudal lung lobe was visualised and found to be adhered to the surrounding mediastinum.
A second ipsilateral thoracotomy was performed at the ninth ICS to breakdown the adhesions and allow for the mass to be exteriorised safely. Adhesions were broken down using a Ligasure device (Figure 5) and lung lobectomy was performed using a 45mm TA blue stapler (45mm x 3.8mm staples) (Figure 6).
The thoracic cavity was lavaged and filled with sterile saline solution to inspect for post-operative air leakage from the lung lobectomy site. A 14-gauge chest tube was placed at the level of the fifth ICS and the chest cavity, subcutaneous tissue and skin were closed routinely. A three-way stopcock was connected to the chest tube and air was then evacuated from the thoracic cavity via a 20ml syringe until negative pressure was obtained. Two non-woven, adhesive wound dressings (Primapore) were applied to cover the incision sites for the first 24 hours. The mass was sent off for histopathologic evaluation.
Post-operative course
The patient underwent an uneventful post-operative recovery and was discharged from the hospital 48 hours after surgery. Residual pneumothorax, pleural effusion and associated hypoxaemia are possible consequences of thoracotomy procedures and were carefully monitored for during the post-operative period. Thoracic pain should always be addressed as well, as it can be a main contributor to hypoxaemia by preventing full thoracic wall excursion.
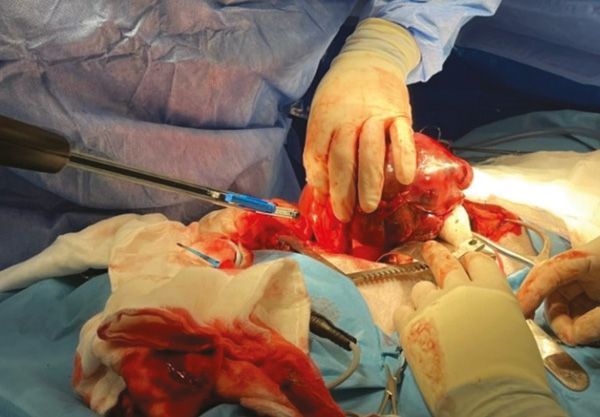
Figure 6: Lung lobectomy using a 45mm TA blue stapler.
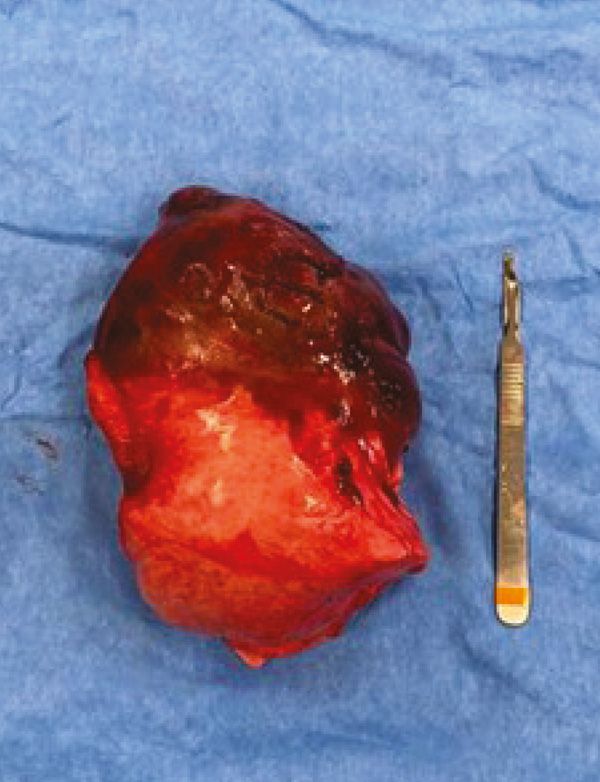
Figure 7: Right caudal lung lobe and associated mass.
For the first 12 hours post-surgery, the patient was on a maintenance fluid rate and methadone CRI.
Pain was then reassessed and given the patient’s increasing comfort levels, methadone CRI was stopped and intravenous buprenorphine administration was started. Fluids were also stopped once the patient went back eating and drinking on its own. The chest tube was checked hourly for the first four hours post-surgery and every four hours thereafter _ to make sure no residual pneumothorax nor pleural effusion were present _ and was removed 24 hours postoperatively. At the time of discharge, the patient was bright, alert and responsive, vitals were within normal limits and breathing pattern and lung sounds were normal.
Conclusion
At the two-week postoperative check, owners reported that the patient had been more energetic and that lameness, ocular discharge and scleral hyperaemia had resolved. Ocular discharge and episcleral injection have been described as a common finding associated with HO. In one study 23/30 dogs with HO displayed ocular signs: most of them were not suffering from any ocular pathology before developing HO.13 The pathological process behind this peculiar presentation remains unknown.13
Histopathological results of the mass was a poorly differentiated soft tissue sarcoma. These tumours carry a high potential for local post-surgical recurrence. In this case, metastatic potential was difficult to predict due to the poor differentiation of the neoplasia.
Survival times for animals showing primary pulmonary neoplasia vary greatly and can go from 26 to 545 days, depending on clinical signs and metastatic spread.9 This dog was considered to have a good prognosis given that this was a solitary mass and no malignant plural effusion, lymph node involvement or metastatic disease were detected at the time of surgery.9 However, the poor differentiation of the neoplasia and its size (i.e., being bigger than 5cm) are negative prognostic indicators.9 Moreover, despite CT dramatically improving detection of metastatic disease when compared to radiographs, it can still be associated with false-negative results.9 These tumours can also be highly infiltrative and, given the initial tumour location and difficulty of excision, failure to obtain clear margins cannot be excluded. For these reasons, owners were strongly advised to perform a recheck CT, ideally within the following six-12 months, to allow for monitoring for tumour recurrence and/or metastatic disease.
To the authors’ knowledge, this is the first reported case of hypertrophic osteopathy secondary to a primary, high grade soft tissue sarcoma of the lung in a dog.
- Costello M. The male reproductive system. In: O'Brien R, Barr F, editors. BSAVA Manual of Canine and Feline Abdominal Imaging. Gloucester: British Small Animal Veterinary Association; 2012. p. 241.
- Doyle E, Hall H, Hughes J, Owen L, Giuliano A. Hypertrophic osteopathy and suspected subsequent disseminated intravascular coagulation in a dog with an abdominal gossypiboma. Veterinary Record Case Reports. 2022; 10(3). Available from: https://doi.org/10.1002/vrc2.394.
- Hammond G, McConnell F. Radiology of the appendicular skeleton. In: McConnell JF, Holloway A, editors. BSAVA manual of Canine and Feline Radiography and Radiology: A Foundation Manual. Gloucester: British Small Animal Veterinary Association; 2013. p. 256
- Lascelles BD, White RN. Tumours of the respiratory system and thoracic cavity. In: Dobson JM, Lascelles BD, editors. BSAVA Manual of Canine and Feline Oncology. 3rd ed. Gloucester: British Small Animal Veterinary Association; 2011. p. 273-274.
- Llabres-Diaz F, Petite A, Saunders J, Schwarz T. The thoracic boundaries. In: Schwarz T, Johnson V, editors. BSAVA Manual of Canine and Feline Thoracic Imaging. Gloucester: British Small Animal Veterinary Association; 2009. p. 365-366.
- Mantis P, Johnson V, Morandi F. The bronchial tree. In: Schwarz T, Johnson V, editors. BSAVA Manual of Canine and Feline Thoracic Imaging. Gloucester: British Small Animal Veterinary Association; 2009. p. 238.
- McAllister H, Tobin E. Long bones – mature. In: Kirberger RM, McEvoy FJ, editors. BSAVA manual of Canine and Feline Musculoskeletal Imaging. 2nd ed. Gloucester: British Small Animal Veterinary Association; 2016. p. 129.
- Mellanby R. Paraneoplastic syndromes. In: Dobson JM, Lascelles BD, editors. BSAVA Manual of Canine and Feline Oncology. 3rd ed. Gloucester: British Small Animal Veterinary Association; 2011. p. 38.
- Monnet E. Lungs. In: Johnston SA, Tobias KM, editors. Veterinary surgery: Small animal. St. Louis: Elsevier Saunders; 2012. p. 1763.
- Nicoll R, Allan G, Davies S. Distal limbs – carpus/tarsus and distally. In: Kirberger RM, McEvoy FJ, editors. BSAVA manual of Canine and Feline Musculoskeletal Imaging. 2nd ed. Gloucester: British Small Animal Veterinary Association; 2016. p. 272-273.
- Towle HA, Breur GJ. Miscellaneous Orthopedic Conditions. In: Johnston SA, Tobias KM, editors. Veterinary surgery: Small animal. St. Louis: Elsevier Saunders; 2012. p. 1122-1123.
- Trumpatori BJ, White RAS. Tumours of the oesophagus. In: Dobson JM, Lascelles BD, editors. BSAVA Manual of Canine and Feline Oncology. 3rd ed. Gloucester: British Small Animal Veterinary Association; 2011. p. 206.
- Withers SS, Johnson EG, Culp WTN, Rodriguez Jr CO, Skorupski KA, Rebhun RB. Paraneoplastic hypertrophic osteopathy in 30 dogs. Veterinary and Comparative Oncology. 2013;13(3):157–165. Available from: https://doi.org/10.1111/vco.12026.
- Yao Q, Altman RD, Brahn, E. Periostitis and hypertrophic pulmonary osteoarthropathy: Report of 2 cases and review of the literature. Seminars in Arthritis and Rheumatism. 2009;38(6):458–466. Available from: https://doi.org/10.1016/j.semarthrit.2008.07.001.
1 Marie’s disease is most often secondary to:
A. A mass in the thoracic and/or abdominal cavity
B. Pulmonary hypertension
C. Chronic kidney disease
D. Congestive heart failure
2. Marie’s disease is usually characterised by the following bone pathology:
A. Pathologic fractures at the distal extremities and long bones
B. Periosteal reaction at the distal extremities and long bones
C. Periosteal reaction at the proximal extremities and long bones
D. Osteoporotic changes at the proximal extremities and flat bones
3. The cranial nerve suspected to have an implication in eliciting the periosteal reaction seen with Marie’s disease is:
A. Facial nerve (CN VII)
B. Optic nerve (CN II)
C. Vagus nerve (CN X)
D. Trigeminal nerve (CN V)
4. When dealing with primary pulmonary neoplasia, an important negative prognostic factor is:
A. Presence of persistent inspiratory effort
B. Presence of persistent expiratory effort
C. Chronic cough
D. Malignant pleural effusion
5. True or false: bone pathology secondary to HO always spares the joint spaces.
A. False: bone pathology secondary to HO always crosses the joints
B. True: bone pathology secondary to HO never crosses the joints
C. False: joint spaces are the primary anatomical region to be affected by HO
D. True: patients affected by HO don’t develop bony changes affecting the joint spaces, but they develop suppurative synovitis instead
ANSWERS: 1A; 2B; 3C; 4D; 5B.














