Allergy and immunoglobulin E – an evolving understanding of the relationship
Allan Bell BVSc, MACVSc, FACVSc, Specialist Veterinary Dermatologist and Boyd Jones BVSc, FACVSc, DECVIM-Ca, Emeritus Professor Small Animal Clinical Studies, University College Dublin, discuss the role of IgE and other mediators in allergic skin diseases
Most of us were taught that an allergic reaction occurs with the aggregation of high affinity IgE receptors (FcεR1) on mast cells and basophils after crosslinking of IgE molecules by multivalent allergens. This sequence of events results in mast cell degranulation with release of inflammatory mediators. Intradermal and serum ‘allergy’ tests were developed to detect IgE on mast cells and in serum to enable identification of relevant allergens. Although these tests might be expected to distinguish allergic cats and dogs from their non-allergic counterparts, they do not. Many healthy cats and dogs have positive intradermal and serum tests based on detection of IgE.
Various serological techniques are used to capture allergen-specific IgE, employing monoclonal or polyclonal anti-IgE and the more recent use of the α chain of the IgE receptor. Refinement of these techniques has helped reduce the spurious identification of IgG as IgE but the discrepancy between positive IgE-based tests and the presence of clinical signs of allergy remains. Such tests have, however, proved valuable for the selection of allergens to include in subcutaneous or oral immunotherapy which remain the only treatments avoiding pharmaceutical agents.
Despite immunological diagnostic tests, the diagnosis of feline and canine atopic dermatitis remains based on clinical assessment followed by the ruling-out of other causes of dermatitis such as infection and parasites rather than reliance on IgE-based tests. Why is this? The following is a brief review of the last 30 years’ research that offers some possible answers and describes some of the discoveries that have led to a more sophisticated understanding of type 1 hypersensitivity.
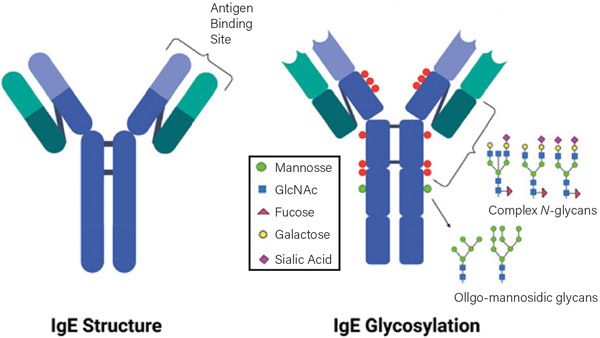
Figure 1. Human IgE showing sites and types of glycans binding on the constant regions (blue segments) of the heavy polypeptide chains. Binding to the high affinity receptor FcεR1 occurs about the junction of C3 and C2 domains. The glycosylation pattern of canine IgE is likely to be different. Figure courtesy of Sagar Aryal (https://microbenotes.com/author/sagararyalnepal/)
Evolution of research
The first indication that IgE was a bit more complicated than first thought came from Lichtenstein (1988). His contribution was a serendipitous discovery while reviewing the results of two research projects involving human histamine-releasing factors. His paper was a remarkable exercise of logical thought, a broad appreciation of allergy, and inspired thinking. The paper is a story of discovery rather than the sparse expression of facts favoured by editors of most scientific journals. The sentence structure is excellent and it is a pleasure to read. He found evidence supporting human IgE (hIgE) functional heterogeneity, raising the possibility that some IgE immunoglobulins might be pathogenic and some non-pathogenic (or less pathogenic). This raises the question of interpretation of allergy tests. Do the serum allergy tests identify pathogenic IgE, less pathogenic IgE or both? Does the identification of non-pathogenic IgE help explain the number of humans, dogs and cats that are not allergic, yet which have positive tests?
Glycosylation of the Fc regions of antibodies (Figure 1) is a post-translational modification by the addition of polysaccharide complexes. These modifications in hIgG define effector functions and have disease-specific patterns. The composition of these glycans affects the outcome of several diseases in humans including rheumatoid arthritis and response to influenza vaccination but the mechanisms are unknown. Several investigators thought that differences in glycosylation might explain the differences in IgE isotypes but Kleile-Tebbe et al (1996) found no evidence to support this theory.
Enough of humans for the moment. Halliwell, our indefatigable researcher in veterinary immunology and dermatology, obtained strong evidence for functional heterogeneity of feline IgE and possible heterogeneity of either canine (c)IgE or its receptor (Halliwell et al, 1998). Briefly, his team employed ELISA testing for Dermatophagoides farinae (Df) (a house dust mite) specific IgE in healthy, atopic and laboratory-reared dogs and cats. Intradermal allergy testing was also performed on all groups except the healthy pet cats. They found: that healthy pet cats and dogs commonly had serum concentrations of house dust mite-specific IgE that did not differ significantly from the atopic groups; that most of the healthy dogs had positive intradermal skin tests to house dust mites; and that the laboratory-reared cats and dogs had very low serum concentrations of house dust mite IgE and negative intradermal tests. The ELISA testing of all groups for house dust mite IgE concentrations was performed using polyclonal antisera that were shown not to identify IgG. Halliwell concluded that heterogeneity was a possible explanation and supported this conclusion with reference to work of Jackson et al (1996) and Peng et al (1997). Jackson showed that basophils from atopic dogs treated with (c)IgE-antisera released more histamine than those from healthy dogs despite there being no significant difference between the groups in either the amount of histamine contained in each basophil or of serum IgE concentrations.
Peng et al (1997) had previously identified two distinct isotypes of IgE from dogs allergic to ragweed. Both isotypes could produce a positive PCA (passive cutaneous anaphylaxis), could be destroyed by heating serum to 56°C, had the same molecular weight and reacted to rabbit anti-dog IgE; but they differed in a significant number of characteristics. These included reacting differently to two types of monoclonal antibodies that were anti-dog IgE, differential binding to protein A (protein A has been used to differentiate the isotypes of IgG, i.e., IgG1, 2 and 4) and having differing isoelectric focusing points on immunoblotting (evidence of physicochemical heterogeneity). Halliwell’s demonstration of functional heterogeneity of canine IgE complements Peng’s finding of physicochemical heterogeneity.
A more recent study of canine IgE heterogenicity (Kumagai et al, 2019 abstract) described preliminary findings that suggested that pathogenic allergen specific IgE bound exclusively to the FccR1 they employed while non-pathogenic IgE to the allergen did not. They could detect the non-pathogenic IgE by heating canine serum then assaying it with a monoclonal antibody in an ELISA. In a second experiment, they obtained ELISA values from three dogs spontaneously sensitised to Df and three laboratory dogs experimentally sensitised to Df. The ELISA was repeated after digestion of the serum with a specific glycosidase and they found the values for IgE increased about five times in the atopic dogs while the values in the experimentally sensitised dogs barely changed. Based on these findings, they suggested the difference in pathogenic and “non-pathogenic IgE is the type of glycosylation (probably high mannose content). We look forward to the results of the proposed larger study to confirm these preliminary findings( Maduska-pers comm).”
Most recently, sialylation (the presence of complex polysaccharides with sialic acid residues) of hIgE has been identified as a determinant of pathogenicity by Shade et al (2020). This group investigated the patterns of glycosylation of hIgE and whether the different patterns detected were associated with different biological activity. They found that IgE from non-atopic people and from individuals with peanut allergy could be distinguished on the basis of glycosylation of the heavy chains of the IgE molecule. HIgE from non-atopics had increased concentrations of terminal galactose while that of atopics had increased terminal sialylation. In addition, they found that the galactose and sialic acid content of hIgE were “uniquely” strong predictors of allergic disease. Serum IgE from atopic individuals produced a significantly greater degree of mast cell degranulation than serum from non-atopic people providing in vitro evidence of functional differences in their respective IgE profiles. Using intravenous Evans blue dye to demonstrate vascular leakage resulted in reduced mast cell degranulation in a mouse ear using sialic-depleted mouse(m)IgE, a finding that could be reversed when sialylation was restored. Importantly, these researchers were also able to demonstrate that despite the functional differences, sialylated and unsialylated mIgE had identical binding affinity to allergens, mast cells and the high affinity IgE receptor. An investigation of the mechanism of this attenuation of mast cell degranulation suggested that without sialylation, an inhibitory glycan on the FccIgE was exposed that dampened FccR1 signalling. These investigators were able to reduce the temperature drop associated with mouse anaphylaxis by treating sensitised mice with sialic residue-free IgE thus raising the therapeutic possibility of blocking the sialic acid on IgE sensitised cells.
To summarise so far:
- Canine, human and feline IgE all show structural, immunological and functional heterogeneity.
- The current serum allergy testing in cats and dogs relates to the total IgE concentration and gives no information about the fractions of isotypes known to have different allergenic properties.
- The diagnosis of allergy might ultimately be based on the ratio of these isotypes rather than their absolute concentrations (Peng et al, 1997).
- Evidence suggests that the difference in ability of isotypes to degranulate mast cells is related to terminal sialic acid residues in human and mouse IgE. This conflicts with Peng’s conclusion that differences in the primary protein structure (of the IgE molecule) were likely to be responsible for the physicochemical differences in the canine isotypes she described.
Our ability to detect these different isotypes would assist our understanding of canine allergies. This might be possible since Peng demonstrated differing immunogenicity in the isotypes she described. We may in the future have an allergy test report that states the concentration of the different isotypes and whether or not these measured values are consistent with a diagnosis of allergy. More speculatively, tests that assay the nature of glycosylation of IgE may become available and enhance our diagnostic ability.
The role of histamine releasing factor
Alas, we have an elephant in the room called histamine releasing factor (HRF). HRF (aka transitional-controlled tumour protein [TCTP] when it is intracellular) is an evolutionarily conserved protein that was molecularly cloned by MacDonald et al (1995). Intracellularly it is required for cell cycle progression, proliferation and cell survival. Platelets, B and T lymphocytes and endothelial cells are all sources of HRF. Little is known about the regulation of HRF but atopic humans have higher serum concentrations than non-atopic individuals (Kashiwakura et al, 2012a). Lichtenstein’s group first identified its association with IgE in humans (MacDonald et al, 1987) and a number of studies have expanded our knowledge of this relationship. Kashiwakura (2012b) have suggested that HRF attaches to the Fab (antigen binding site on immunoglobulins, Figure 1) structure on a subset of hIgE termed IgE+. When this form of IgE binds to human mast cells and basophils they release histamine, interleukin (IL)-4 and IL-13 (Schroeder et al, 1997) from human basophils and IL-13 and tumour necrosis factor (TNF) from murine mast cells (Schroeder et al, 1996). HIgE- is unable to achieve these effects. Based on the above evidence, it seems allergen binding is not required for activation of mast cells and basophils by IgE+. (MacDonald et al, 1987). Serum from atopic humans can activate mast cells in vitro (Kashwakura 2009), underlining the ability of IgE+ to activate mast cells in the absence of allergen.
HRF has two binding sites for IgE+ and dimers and oligomers can aggregate numbers of FcεR1 receptors on mast cells and basophils in the same manner as polyvalent allergens. Murine mast cells have enhanced production of IL-6, IL-13 and TNF in response to IgE alone or from an allergen-IgE complex. Histamine release, on the other hand, seems fully activated by an allergen-IgE complex. These findings (Kawakami et al, 2020) suggested that stronger or more persistent FcεR1 aggregation is required for cytokine production versus mere degranulation. It follows that qualitative and quantitative differences in mast cell and basophil activation may result from a range of possible stimuli including HRF bound to IgE+ with or without allergen and allergen specific IgE- bound to allergen. These different stimuli might ultimately relate to the range of clinical presentations of atopic dermatitis. Heterogeneity of hIgE can be described as: differences in the ability to stimulate cytokine production by mast cells and basophils, differences in ability to bind HRF, speculative structural differences in the IgE molecule (Peng et al, 1997; Buddle et al, 2000) or variations in glycosylation (Kawakami et al, 2020). It might not be too outrageous to consider that some combination of the above might finally define these putative mechanisms.
In summary, HRF bound to a subtype of IgE (IgE+) can activate mast cells and basophils in the absence of allergen. ‘Normal’ concentrations of serum HRF will not support this activation. The regulation of HRF is unknown and is difficult to research because knock-out mice (those without the genes for HRF/TCTP) do not survive. Peptides that block HRF binding to IgE diminish mast cell and basophil activation and mice with induced atopic dermatitis and respiratory allergies improve after binding is prevented. This suggests that agents that block HRF have potential as a treatment for allergies (Kashiwakura et al, 2012b).
Neither heterogeneity of IgE nor HRF provide a ready explanation of false positive intradermal allergy tests to HDM in numbers of healthy dogs and those with induced hypersensitivity but no clinical manifestations. Studies of immunological responses in subjects with helminth infection and concurrent house dust mite exposure have yielded some insights into interactions (Santiago et al, 2015, Junginger et al, 2017).
The “hygiene theory” proposes that allergy is the result of type 2 immune responses normally directed at large foreign organisms such as helminths being misdirected at innocuous environmental antigens. While the mechanism and consequences of this misdirection have not been described in detail some progress has been made. There is good evidence that ascarid infection is responsible for total cIgE values being much higher than those of humans (Griffin et al, 1990, Zwickl et al, 2018). Griffin also demonstrated a positive correlation between the frequency of false positive serum testing and increases in total cIgE concentrations. Halliwell et al (2021) suggested that cross reaction with helminths and mite allergens is responsible for false positive HDM IgE serology in cats.
These authors cited two papers supporting such a relationship between A lumbricoides and mite allergens in other species. Other studies of helminth/mite cross reactions have identified multiple structural homologies between HDM and ascarid larvae (Gazzinelli-Guimaraes et al,2021, Doyen et al, 2021). The relevance of the identified cross reaction is debated as they rarely involve the antigens of Df known to be involved in canine HDM allergy. Doyen et al (2021) found IgE induced during helminth infections in humans is not associated with allergic symptoms and suggested the correlation between total IgE to allergens and ascarid specific IgE “is a non-specific polyclonal stimulation effect: a class effect rather than a specific allergen sensitisation induced by helminth infection”. They observed “there is little known about factors that modulate the ability of IgE to trigger mast cell degranulation during helminth infections”. In contrast, Favrot et al (2021) considered that Toxacara canis infection induced HDM hypersensitivity in 3/6 of their laboratory dogs. No explanation was offered for an uninfected ‘control’ dog showing similar HDM positivity (Fischer et al, 2018).
There is insufficient evidence as yet to support helminths in initiating HDM allergy in healthy dogs or modifying clinical signs of established HDM allergy. An interim conclusion might be that helminth infections do affect total cIgE and HDM IgE serum values.
A further cause of false positive serological tests may be allergen specific IgE to cross reactive carbohydrates (CCD) present on the glycoprotein allergen, rather than to a peptide antigen. CCD specific IgE are common in pollens in particular and are regarded as non-pathogenic in humans. This is not universally so, as Favrot (2021) reminded us of the role of alpha-galactosidase in meat allergy. Levy and DeBoer (2018) have identified IgE to CCDs in dogs and Lee et al (2020) have demonstrated the use of blockers that remove this contribution to specific IgE serum values in cats and dogs.
Glycosylation and IgE isotypes of cats, dogs and horses as well as their interaction with HRF deserve further scrutiny. We need confirmation that HRF is a contributor to the clinical manifestations of allergy in our patients. This is likely because HRF is evolutionarily conserved. The relevance of glycosylation also needs to be determined. Our understanding of IgE heterogeneity in dogs might be clarified by repeating Peng’s study using IgE+/- to investigate possible similarities to the isotypes she called IgE1 and IgE2.
In the case of immunological interactions between helminths and HDM, Santiago et al (2016) suggested the effect helminths have on canine IgE production to HDM may approximate a case of the “original antigenic sin” whereby the immune system reacts to an antigen with significant homology to an antigen to which it has already created memory cells rather than produce a new primary response. This idea appeals so watch this space, there is more to come.
Acknowledgement
A version of this paper was published in Companion Quarterly 2021;31(4);12-14, the official newsletter of the Companion Animal Branch of the New Zealand Veterinary Association and is re-published with the permission of the editor.
- Buddle IK, Aalbers M, Aalberse RC et al. Reactivity to IgE-dependent histamine-releasing factor is due to monomeric IgE. Allergy 2000; 55: 653–57
- Doyen V, Truyens C, Thi HN et al. Helminth infection induces non-functional sensitisation to house dust mites. PLoS One 2021;16, (7): 30253887
- Favrot C, Jacquence S, Fischer N et al. Toxocara canis infestations influence seroconversions to Dermatophagoides farinae IgE. Proceedings of the 29th Annual Congress of the ESVD-ECVD (poster) 2021.
- Fischer N, Rostaher L, Zwickl L et al. A Toxocara canis infection influences the immune response to house dust mite allergens in dogs. Veterinary Immunology and Immunopathology 2018; 202: 11-17
- Gazzinelli-GuimaraesPH, Bennuru S, Prado R et al. House dust mite sensitization drives cross-reactive immune responses to homologous helminth proteins. PLoS Pathology 2021;17(3): e1009337
- Griffin CE, Moriello KA, DeBoer DJ. The effect of Serum IgE on an in vitro ELISA test in the Normal Canine. In Von Tscharner C and Halliwell REW eds. Advances in Veterinary Dermatology 1, Balliere Tindall.137-44, 1990
- Halliwell REW, Gilbert SM, Lian TM. Induced and spontaneous IgE antibodies to Dermatophagoides farina in dogs and cats: evidence of functional heterogeneity of IgE. Veterinary Dermatology 1998;9:179–84
- Halliwell R, Banovic F, Mueller R, Olivry,T. Immunopathogenesis of the feline atopic syndrome. Veterinary Dermatology 2021; 32:13-25
- Jackson HA, Miller HRP, Halliwell REW. Canine leucocyte histamine release: response to antigen and to anti-IgE. Veterinary Immunology and Immunopathology 1996;53: 195–206
- Kashiwakura J, Kawakami Y, Yuki K et al. Polyclonal IgE induces mast cell survival and cytokine production. Journal of Investigational Allergology and Clinical Immunology, 2009;58: 411-19
- Kashiwakura J, Okayama Y, Furue M, Kabashima K, Shimada S, Ra C, Siraganian RP, Kawakami Y, Kawakami T. Most cytokinergenic IgEs have polyreactivity to autoantigens. Allergy Asthma and Immunology Research 4,33240, 2012a
- Kashiwakura JC, Ando T, Matsumato K et al. Histamine releasing factor has a proinflammatory role in mouse models of asthma and allergy. Journal of Clinical Investigation 2012b;122:218–28
- Kleile-Tebbe J, Kagey-Sobotka A. Lectins do not distinguish between heterogeneous IgE molecules as defined by different activity of an IgE-dependent histamine-releasing factor. Journal of Allergy and Clinical Immunology 1996;98: 181–8
- Kumagai A, Saitama M, Tsuku T and Madusa K. Two types of IgE in dog serum differ in glycosylation. Veterinary Dermatology 2019; 30: 462-3
- Lee KW, McKinney BH, Blankenship KD, Morris DO. Detection and Inhibition of IgE for cross-reactive carbohydrate determinants evident in an enzyme-allergen-specific IgE in the sera of dogs and cats. Veterinary Dermatology 2020:31(6): 439-e116
- Levy BJ and DeBoer DJ. A preliminary study of serum IgE against cross-reactive carbohydrate determinants CCD in client owned dogs. Veterinary Dermatology 2018;29(3):243-e90
- Lichtenstein LM. Histamine releasing factors and IgE heterogeneity. Journal of Allergy and Clinical Immunology 1988;81: 814–20
- MacDonald SM, Lichtenstein LM, Proud D et al. Studies of IgE-dependent histamine releasing factors: Heterogeneity of IgE. Journal of Immunology 1987;139: 506–12
- MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM. Molecular identification of an IgE-dependent histamine-releasing factor. Science 1995;269 688–90
- Peng Z, Arthur G, Rector ES et al. Heterogeneity of polyclonal IgE characterized by differential charge, affinity to protein A, and antigenicity. Journal of Allergy and Clinical Immunology 1997;100: 87–95
- Santiago H, Ribeiro-Gomes FL, Bennuru S, Nutman TB. Helminth infection alters IgE responses to allergens structurally related to parasite proteins. Journal of Immunology 2015:194(1):93-100
- Shade KC, Conroy ME, Washburn N et al. Sialylation of immunoglobulin E is a determinant of pathogenicity. Nature 2020;582: 265–70
- Schroeder JT, Lichtenstein LM, MacDonald SM. An immunoglobulin E-dependent recombinant histamine-releasing factor induces interleukin-4 secretion from human basophils. Journal of Experimental Medicine 1996;183:1265–70
- Schroeder JT, Lichtenstein LM MacDonald SM. Recombinant histamine-releasing factor enhances IgE dependent IL-4 and IL-13 by human basophils. Journal of Immunology 1997;159: 447–52
- Zwickl L, Joekl, DE, Fischer NM et al. Total and Toxocara canis larval excretory/secretory antigen- and allergen-specific IgE in atopic and non-atopic dogs. Veterinary Dermatology 2018;29:222-e80
Suggested Reading
Kawakami T, Kashiwakura J, Kawakami Y. Histamine releasing factors and immunoglobulins in asthma and allergy. Allergy Asthma and Immunology Research 2014;6:6–12.
This paper summarises most of the in vivo and in vitro scientific work on HRF and IgE in mice and humans up to 2014.
Kawakami Y, Kasakura K, Kawakami T. Histamine-releasing factor, a new therapeutic target in allergic diseases. Cells, 2019;8:1515
A review article describing newer advances in the HRF/IgE relationships. It has a table summarising treatments that have modulated murine disease models and human patients with atopic dermatitis food allergy etc.
1) Functional heterogeneity of IgE is associated with:
A. Glycosylation of the Fc region
B. The complement binding region
C. The hinge region
2) Serum-based tests for which allergen are least accurate in dogs?
A. Plant pollen
B. House dust mite
C. Food allergens
D. Flea antigens
3) Antigen specific cross reactive carbohydrate IgE is considered pathogenic:
A. True
B. False
4) How might ELISA testing for pollen allergens be made more accurate?
A. Treat the dog with an anthelmintic prior to testing
B. Treat with an antihistamine before testing
C. Use a CCD blocker prior to testing
5) Histamine releasing factor has been well researched in dogs?
A. True
B. False
6) Which parasite is associated with the discrepancy between the total IgE concentrations measured in humans and dogs?
A. Uncinaria stenocephala
B. Fleas
C. Demodex canis
D. Toxocara canis
Answers: 1A; 2B; 3 False; 4C; 5 False; 6D.









