Small animal - September 2020
Chronic kidney disease in cats – part 1
Vanessa Bourne MVB CertAVP(SAM-F) PGCertVetEd FHEA MRCVS, Royal Veterinary College, discusses how to increase longevity, improve the client-practice bond and increase revenue by employing optimal monitoring and treatment options for chronic kidney disease in cats
Chronic kidney disease (CKD) is a very common finding in mature cats, potentially affecting over 30% of cats over 10 years of age. CKD is a generally slowly progressive disorder, caused by chronic tubulointerstitial nephritis and interstitial fibrosis in most cases. The underlying cause remains undetermined but may be related to being affecting by hypoxia, chronic glomerulonephritis, pyelonephritis, upper urinary tract obstructions or viruses. To consider a patient is suffering from CKD, there should be evidence of persistent loss of renal function for over three months.
Given that cats can survive for many years with CKD, it makes good business sense for clinicians to become familiar with the optimal monitoring and treatment options. Monitoring patients can also form the basis of a geriatric cat clinic. Increased interaction with cat and owner improves the practice-client bond and is likely to increase revenue in the long run. Clinicians should, therefore, make an effort to make their premises feline-friendly and ensure all personnel have received training in feline-friendly handling techniques. Owners are increasingly using the internet to seek out cat-friendly clinics and satisfied owners are likely to promote practices where they have had a good experience. Good previous visits and positive social-media reviews encourage owners to bring their cats to the clinic.
CKD is a complex disease that is the end result of a wide range of possible disorders affecting the kidney, where irreversible damage has occurred leading to a reduction in glomerular filtration rate (GFR). Multiple factors may be involved in the development of CKD, including genetics, age, individual and environmental factors. Certain breeds may be predisposed to CKD, for example Persian, Abyssinian, Siamese, Ragdoll and Maine coon cats. In some cases, a specific cause can be identified, for example a known previous toxic insult (lily ingestion, non-steroidal anti-inflammatory drug administration), neoplasia, polycystic kidney disease, amyloidosis or hypercalcaemia. In many cases no primary cause can be identified. In these cases, chronic tubulointerstitial nephritis and interstitial fibrosis are commonly present. These histological findings are likely to be due to a degenerative process, which may be caused by repeated renal hypoxia (for example, animals undergoing general anaesthesia), exposure to toxins, glomerulonephritis or pyelonephritis among other factors. Animals receiving annual or frequent vaccinations may have an increased risk of developing CKD. Feline viruses are initially propagated using an immortal line of feline-derived tubular epithelial cells during vaccine manufacturing. Exposure to antigenic components can occur and in theory antibody production in the recipient may be stimulated. This could bind feline renal proteins and initiate and inflammatory response. Periodontal disease can produce a chronic systemic inflammatory response as well as local inflammation and has been proposed as a potential cause of CKD. Cardiovascular disease in humans is known to increase an individual’s risk of developing CKD. Research is on-going as to whether cardiovascular disease may play a role in the development of CKD in feline patients.
Common findings in patients with CKD include weight loss, polyuria and polydipsia (PUPD), abnormal renal palpation, dehydration or hypertension. Urine specific gravity (USG) concentration is affected once two-thirds of the nephrons are dysfunctional. Therefore, detection of USG less than 1.035 in cats should prompt further investigation or monitoring. Azotaemia does not occur until three-quarters of nephrons have been affected. Recently, symmetric dimethyl arginine (SDMA) has been investigated as a potentially useful biomarker in CKD. Concentrations begin to rise once 40% of nephrons have been compromised. Therefore, SDMA is a biomarker that has been proposed to diagnose CKD at an earlier stage than creatinine, potentially allowing earlier interventions. Research is currently on going into its sensitivity and specificity, and the influence of extrarenal diseases.
Table 1: IRIS Staging of CKD using serum creatinine and SDMA concentrations.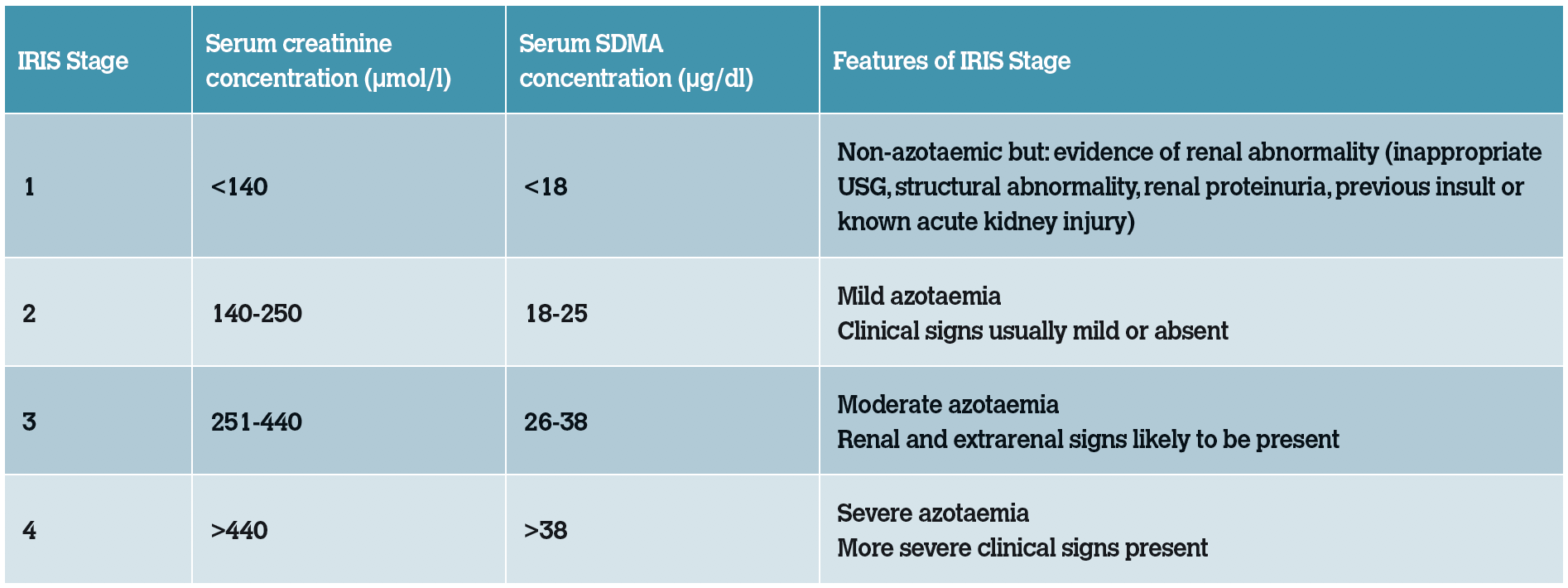
Clinical signs observed in cats with CKD
- PUPD
- Weight loss or reduced body condition
- Dehydration
- Hypertension
- Abnormal renal palpation
Currently creatinine is used as a biomarker to approximate glomerular filtration rate (GFR). Creatinine is a by-product of creatinine phosphokinase. It has an exponential relationship with GFR, meaning that significant early declines in GFR produce a small increase in creatinine, while in later disease larger increases in creatinine may only reflect small decreases in GFR. Urea can also be used in assessment of CKD, however this is more likely to be affected by non-renal factors than creatinine. Urea is a metabolite from ammonia absorbed in the intestine and transported to the liver. Therefore, it can be affected by protein intake and hepatic or intestinal dysfunction.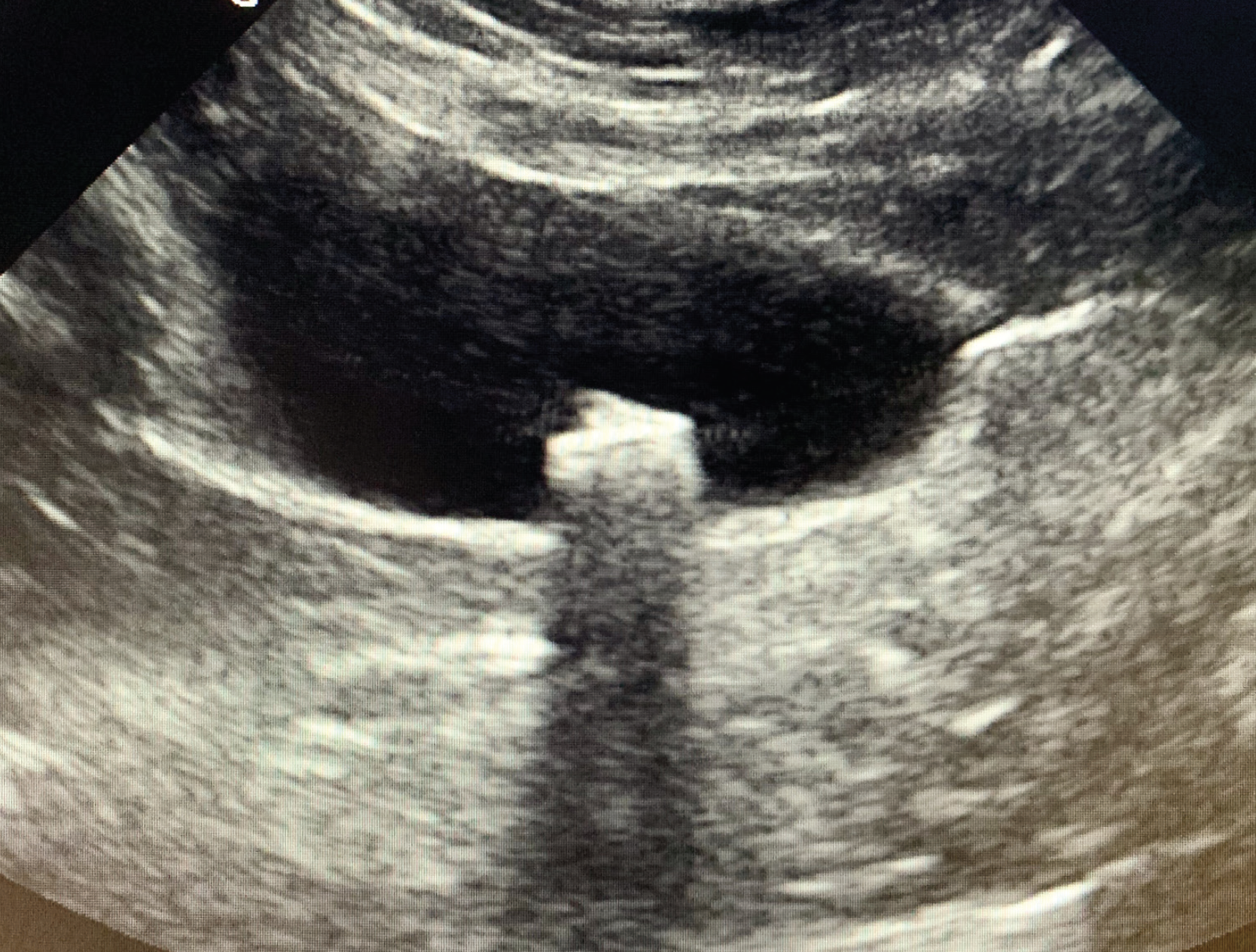
Figure 1: Diagnostic imaging can be beneficial in diagnosing concurrent conditions, such as uroliths.
Diagnosis of CKD in feline patients
In practice, CKD is generally diagnosed on the presence of: an increased serum creatinine concentration (>140 µmol/l) and evidence that clinical findings have been present for a significant length of time (>3 months). There are some exceptions to this. Some cats will have evidence of structural renal damage noted on diagnostic imaging or renal proteinuria without changes in USG or azotaemia. Some animals will have a persistent reduction in USG for some time before developing azotaemia. Development of an increase in serum creatinine concentrations (>15%) which is persistent, even if non-azotaemic, is likely to indicate a reduction in renal function.
The International Renal Interest Society (IRIS) has developed a number of monitoring and treatment recommendations, allowing for clear guidelines and interventions. Patients are currently staged according to serum creatinine levels and then sub-staged based on blood pressure and proteinuria measurements. Creatinine should always be measured on a stable, fasted, hydrated patient to be considered valid and measured on more than one occasion to demonstrate persistence at that level. The Staging Guidelines have recently expanded to include SDMA values.
It has been proposed that SDMA may be more sensitive as it is less impacted by changes in lean body mass. Therefore, the following recommendations have been made:
If serum or plasma SDMA is persistently >18 µg/dl in a cat that is classified as IRIS CKD stage 1 based on creatinine, this feline patient should be staged and treated as an IRIS CKD stage 2 patient;
If serum or plasma SDMA is persistently >25 µg/dl in a cat that is classified as IRIS CKD stage 2 based on creatinine, this feline patient should be staged and treated as an IRIS CKD stage 3 patient; and
If serum or plasma SDMA is persistently >38 µg/dl in a cat that is classified as IRIS CKD stage 3 based on creatinine, this feline patient should be staged and treated as an IRIS CKD stage 4 patient.
IRIS substaging of CKD based on proteinuria
Proteinuria in cats with CKD is associated with a poorer prognosis. The magnitude of proteinuria should be assessed by measurement of urine protein:creatinine ratio (UPC). Urine protein dipstick reagent pads are insensitive and can produce false results. Therefore, they are not recommended to diagnose or monitor progression of proteinuria. UPC assessment involves measuring the urinary protein and creatinine concentrations and calculating the ratio of analyte concentrations. This provides an accurate quantification of the amount of protein excreted in the urine. The values can be used to determine whether the animal is proteinuric, borderline proteinuric or non-proteinuric and whether intervention by the clinician is required. It can also be used to monitor progression of disease and response to treatment.
It is not possible for most standard practice analysers to measure UPC; therefore, this is usually a test that is requested at an external reference laboratory. It can be combined with other urinalysis procedures, for example urine culture, and is a low-cost test.
Proteinuria can be classified as pre-renal, renal or post-renal. Pre-renal proteinuria is associated with abnormal circulating proteins, as might be seen in neoplastic diseases. Post-renal proteinuria can be associated with bladder issues such as a urinary tract infection, or genital tract inflammation if the sample obtained is free-catch. UPC measurement on a sample obtained by cystocentesis is therefore most accurate. Renal proteinuria is associated with proteinuria as a result of kidney disease. Increased intraglomerular capillary pressure and other factors alter glomerular permeability leading to loss of albumin and other proteins into tubular fluid. This proteinuria has been associated with tubular inflammation and fibrosis.
Excluding pre-renal and post-renal causes can identify renal proteinuria. This includes measurement of plasma proteins to exclude dysproteinaemias and urinalysis to exclude haemorrhage or urinary tract inflammation or infection.
Table 2: IRIS substaging of CKD based on proteinuria.
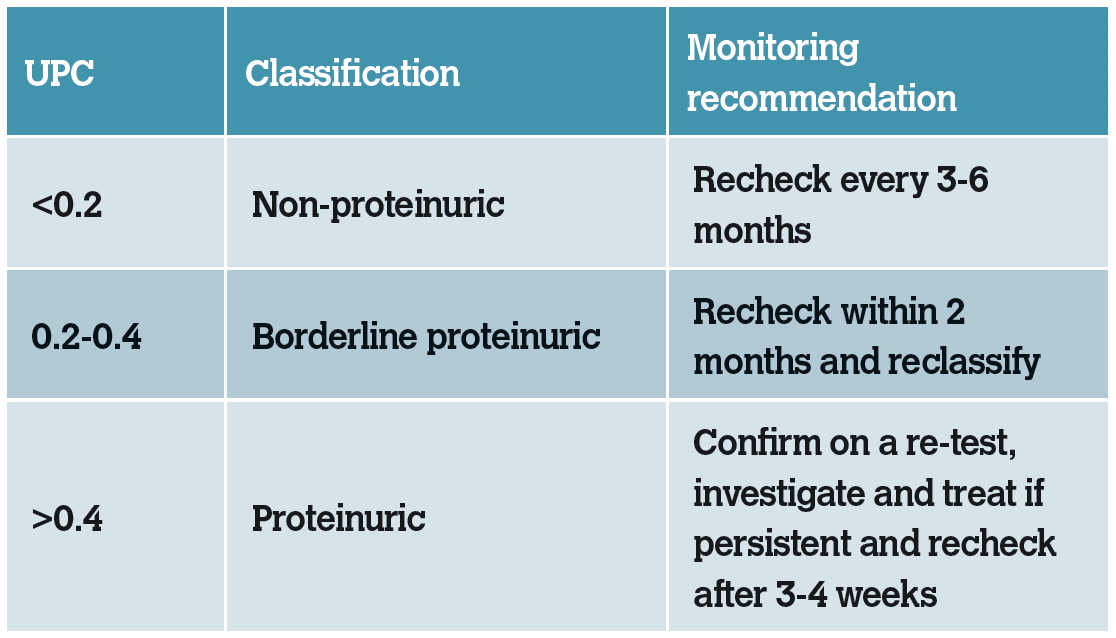
Patients that are borderline proteinuric should be assessed for any concurrent associated diseases present. If no pre- or post-renal diseases are suspected, the UPC should be remeasured within two months to demonstrate persistence or resolution. Treatment of cats in this range with anti-proteinuric measures is controversial and no consensus exists. The individual clinical may wish to treat or continue to monitor.
Cats that are persistently proteinuric (UPC>0.4 on more than one occasion) should be assessed for any concurrent associated diseases present and any detected diseases present should be treated, with subsequent reassessment of proteinuria. Hypertension is a concurrent cause of proteinuria; therefore, blood pressure should always be assessed in patients with CKD. In some cases, a renal biopsy may be indicated, however this would be uncommon. Persistently proteinuric cats should be treated with a renin-angiotensin-aldosterone system (RAAS) inhibitor see Management of CKD in Part 2 and transitioned onto a renal diet (which is restricted in dietary protein).
IRIS substaging of CKD based on blood pressure
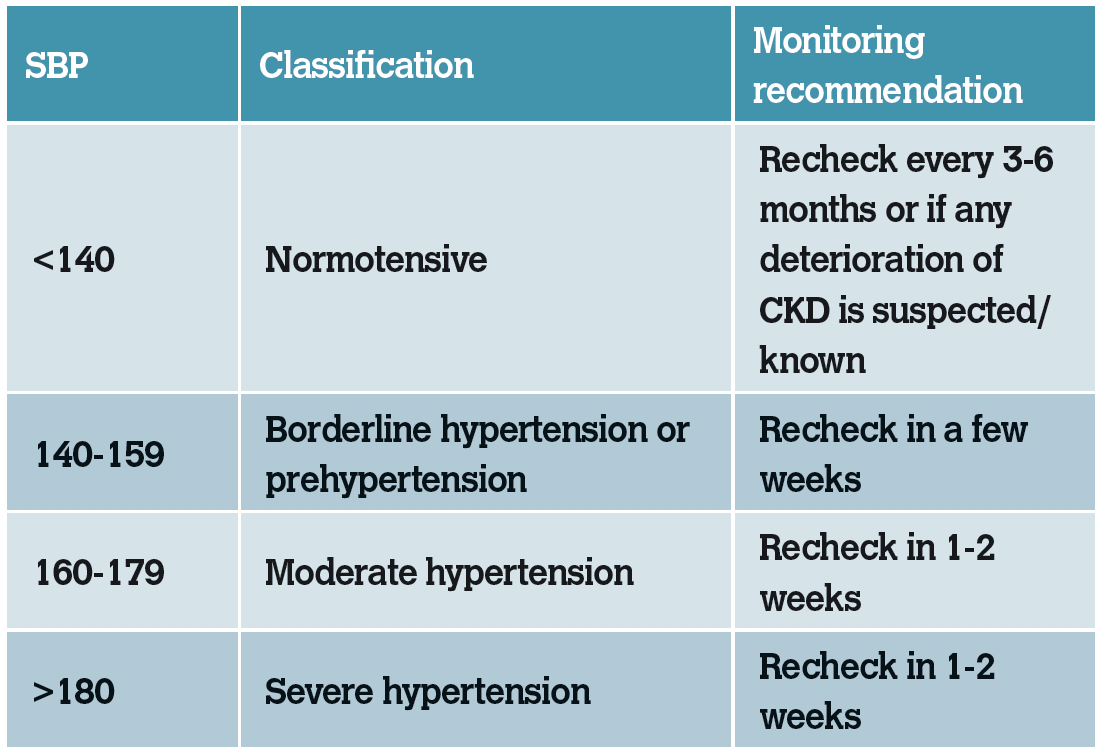
While hypertension is not yet recognised as a negative prognostic indicator in cats with CKD, it has been associated with a worsening of proteinuria. Prolonged hypertension is associated with subsequent target organ damage (TOD) in organs with blood supply. These include the heart, eyes, kidneys and brain. Patients with persistent hypertension should be treated with a calcium channel blocker or angiotensin receptor blocker see Management of CKD in Part 2.
Table 3: IRIS substaging of CKD based on blood-pressure measurement.
Mineral and bone disorder associated with CKD
Development of CKD affects regulation of several solutes excreted in urine, including phosphate and calcium. In the early stages of CKD, hormones are able to adapt and control regulation of calcium and phosphate, maintaining normal plasma levels. In later stages of CKD, these hormones are unable to maintain regulation and calcium and phosphate move outside the reference range despite increasing levels of hormone.
Changes in phosphate and calcium levels occur relatively late in the stages of CKD, however increased plasma concentrations of hormones can be identified earlier in disease and are recognised as early indicators of mineral and bone disorder (MBD) associated with CKD. CKD-MBD is now used to describe what was previously termed secondary renal hyperparathyroidism.
Homeostatic regulation of calcium and phosphorus is complicated; however a basic interpretation can help understand the development of mineral and bone disorder. The body systems that control calcium and phosphorus metabolism are the kidneys, gastrointestinal tract and bone.
Both calcium and phosphate are regulated by parathyroid hormone (PTH) and calcitriol (1,25-dihydroxycholecalciferol). The primary purpose of PTH is to regulate extracellular fluid calcium concentration. PTH is released when a reduction in ionised calcium is detected and acts to restore calcium levels to the reference range. PTH causes both excretion and resorption of phosphate, so exerts competing effects in the kidneys and bone. Calcitonin is released when an increase in ionised calcium is detected and suppresses the secretion of PTH and calcitriol. Raised phosphate levels stimulate the release of PTH while inhibiting calcitriol formation. Fibroblastic growth factor 23 (FGF-23) is secreted from bone in response to an increase in plasma phosphate or calcitriol levels. It is a phosphatonin, whose main function is to decrease plasma phosphate concentrations.
Table 4: Simplified summary of characteristics of hormones involved in calcium and phosphate homeostasis.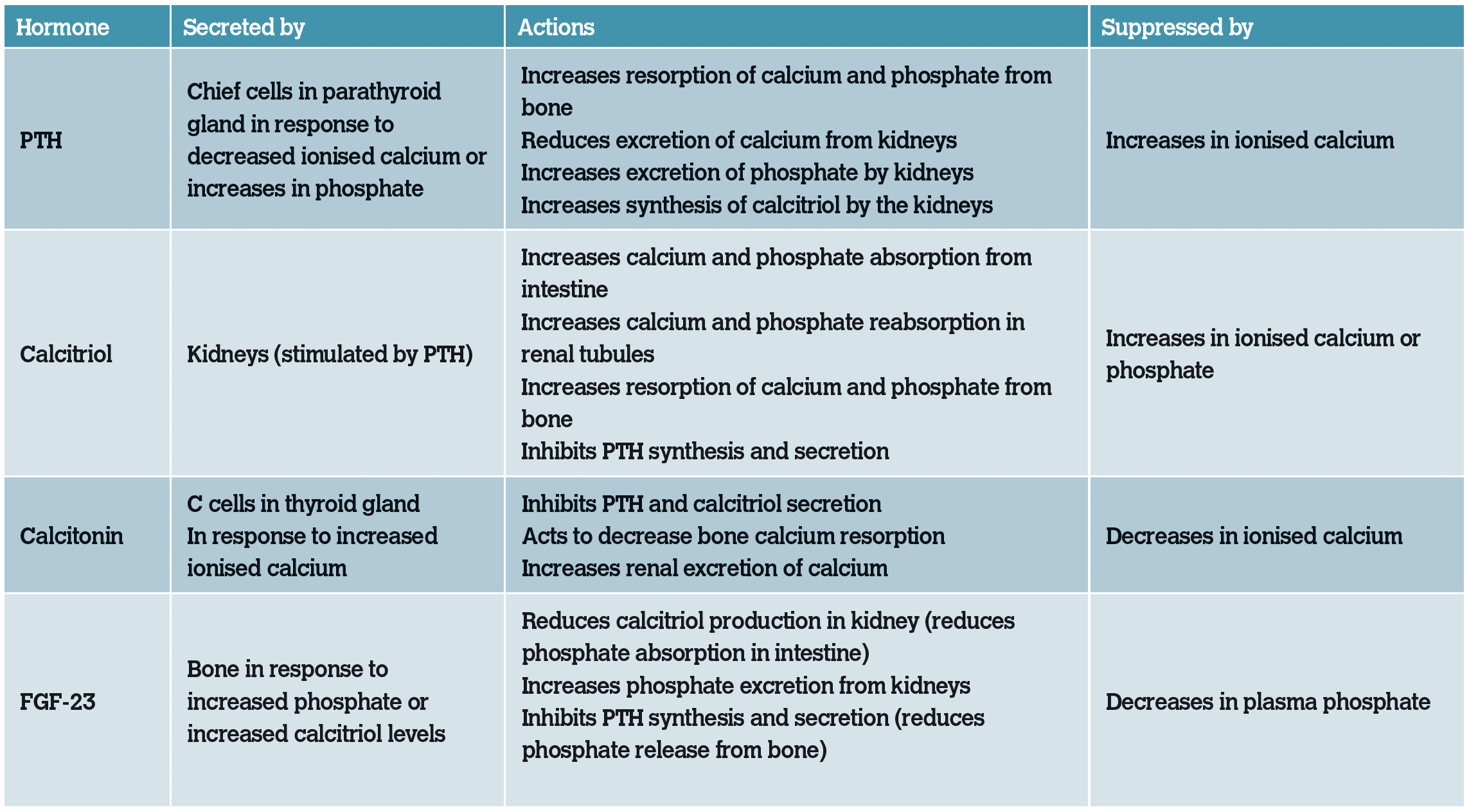
Pathophysiology of CKD-MBD
Phosphate retention develops as GFR decreases in a dysfunctional kidney. In early CKD, an increase in phosphate causes an increase in FGF-23 concentrations. This results in excretion of phosphate, inhibition of calcitriol (decreasing resorption of phosphate from the gut) and PTH (preventing the release of phosphate and calcium from bone). These mechanisms are enough to maintain normal phosphate levels and prevent progression of CKD-MBD.
However, as the renal function declines, these mechanisms become inappropriate. FGF-23 is renally cleared; therefore, it will increase with a falling GFR. End-organ resistance to FGF-23 develops, reducing inhibition of PTH. Elevations in plasma phosphate associated with declining renal excretion stimulates continued release of FGF-23.
FGF-23 continues to suppress calcitriol but becomes less effective at renally excreting phosphate. Reduced calcitriol reduces inhibition of PTH secretion in the parathyroid gland. A reduction in calcitriol reduces calcium and phosphate absorption in the intestine, indirectly increasing PTH secretion. Overall, there is an increase in PTH due to homeostatic mechanisms in response to low ionised calcium concentrations and hyperphosphataemia. There is resistance to FGF-23 at the parathyroid gland, which means PTH secretion is no longer inhibited, despite the on-going elevations in FGF-23.
As CKD progresses to late-stage disease, cats will have elevated FGF-23 concentrations, elevated PTH concentrations along with hyperphosphataemia and a reduction in calcitriol concentrations.
Why is this important?
CKD-MBD is associated with significant morbidity and decreased survival. Bone demineralisation and soft tissue mineralisation can occur. Calcium plays key roles in multiple physiological processes. It is involved in neuromuscular transmission, enzyme activity, coagulation of blood, normal cellular function and muscular contraction, maintaining blood vessel tone and therefore blood pressure. As the main component of the skeleton, it maintains the structural integrity of bones and teeth.
Hyperphosphataemia and hyperparathyroidism are common findings in patients with CKD. Calcium abnormalities are noted less frequently than hyperphosphataemia. Ionised hypocalcaemia is the most prevalent calcium abnormality noted in patients with CKD and tends to occur in later-stage disease. However, total and ionised hypercalcaemia can also occur. Hyperparathyroidism can be present in cats with normal plasma phosphate and ionised calcium levels and can be detected in cats before azotaemia develops. Unfortunately, measurement of PTH is not easy in first opinion practice, which precludes its use as a risk factor for CKD.
Studies have shown that increases in FGF-23 have been associated with a reduction in renal function. This means that FGF-23 may be used as a predictor for the development of CKD-MBD in the future and it may be the earliest predictor in azotaemic patients that have normal phosphate concentrations.
Hyperphosphataemia has been recognised as a negative prognostic indicator in patients with CKD. Detection of Increases of plasma hormones such as FGF-23 and PTH are recognised as earlier indicators of mineral bone disorder seen in CKD. While measurement of plasma phosphate and ionised calcium are commonplace in first opinion practice, measurement of calcitriol, PTH and FGF-23 are more expensive and technically challenging, although this may change in the future.
While hyperphosphataemia occurs late in the development of CKD-MBD, it is a valuable monitoring tool for patients with CKD. It can be used as a biomarker of CKD-MBD using target ranges set by IRIS, rather than the reference range set by external laboratories. This allows earlier interventions to be implemented to try and prevent progression of CKD-MBD. Studies have indicated that restricting phosphate
intake in cats with azotaemic CKD reduces plasma FGF-23 concentrations, therefore monitoring and intervening where recommended may help to potentially reverse changes. The aim should be to maintain a patient’s plasma phosphate concentration at the lower end of the target range or at least within the target range.
Table 6: IRIS recommended target serum phosphate concentrations.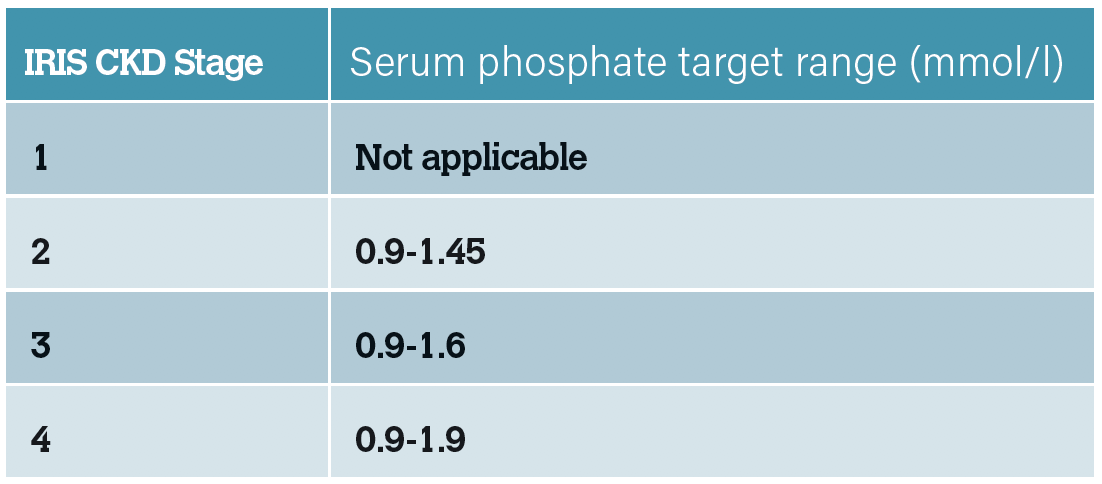
It is recommended to measure plasma phosphate (and creatinine) on fasted samples as both can be affected by food intake. Recently, it has been discovered that magnesium may play a role in morbidity and mortality associated with CKD. In studies, hypomagnesaemia was associated with higher plasma FGF-23 concentrations and an increased risk of death. 
Figure 2: Interventions such as intravenous fluid therapy, medications or tube feeding may be needed to support patients with CKD.
Measurement of total magnesium is possible in first opinion practice and may augment prognostic information in cats with CKD. Further investigations into the prognostic significance of plasma magnesium concentrations in cats with CKD are on going.
The above parameters provide useful information in the staging and monitoring of CKD in cats. Following diagnosis or any treatment interventions, it would be reasonable to reassess relevant parameters after successful treatment and then again at a suitable intervals depending on results. Initial reassessment may be recommended between one and four weeks following diagnosis, depending on the abnormalities detected. Stable cats should be reassessed every three to six months ideally. Full monitoring will not be required at every check. The intervals between examinations possible will vary depending on cat and owner factors.
In conclusion, CKD is a multifactorial disease common in feline patients. With appropriate investigation and monitoring, pets can live a long time with their disease. The next article will focus on management and treatment options available for patients with CKD and associated complications.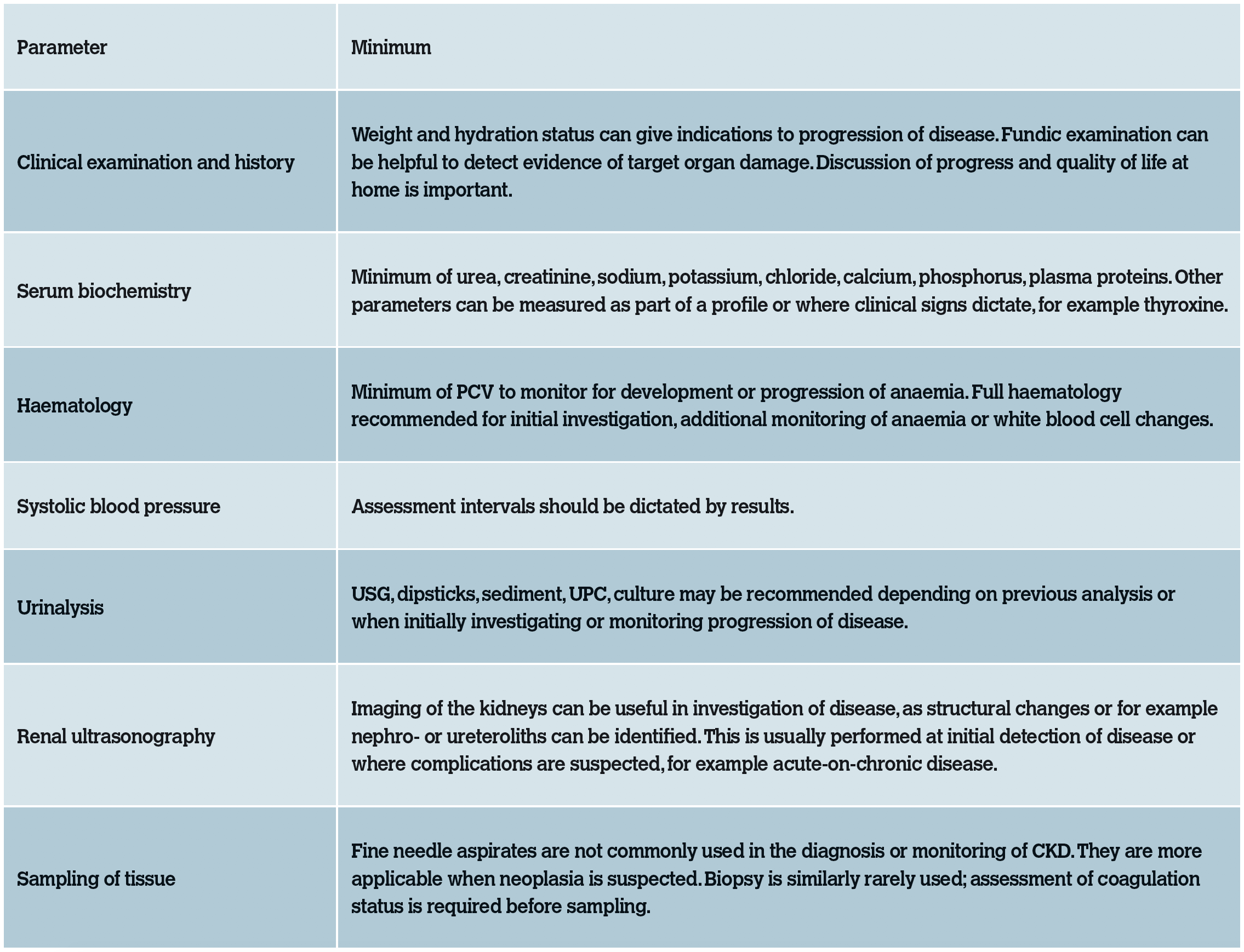
Table 7: Database parameters recommended in the investigation or monitoring of CKD.
-
Finch, NC et al. 2016. Risk factors for development of chronic kidney disease in cats. Journal of Veterinary Internal Medicine 30, pp.602-610.
-
Geddes R, Elliott J. 2017. ‘Assessment of calcium and phosphate homeostasis in chronic kidney disease’, in Elliott, J. Grauer, G.F. Westropp, J.L., eds., BSAVA Manual of Canine and Feline Nephrology and Urology, 3rd ed., Gloucester: BSAVA, pp. 143-150.
-
International Renal Interest Society (2019) ‘IRIS Staging of CKD’, [onlinehttp://www.iris-kidney.com/pdf/IRIS_Staging_of_CKD_modified_2019.pdf [accessed 14th July 2020]
-
Jepson RE. Current understanding of the pathogenesis of progressive chronic kidney disease in cats. Veterinary Clinics of North America, 46, pp. 1015-1048.
-
Olin SJ, Bartges JW. 2015. Urinary tract infections. Veterinary Clinics of North America, 45, pp. 721-746.
-
Sparkes AH, et al. 2016. ISFM Consensus guidelines on the diagnosis and management of feline chronic kidney disease. Journal of Feline Medicine and Surgery, 18, pp. 219-239.
-
Taylor SS, et al. 2017. ISFM Consensus guidelines on the diagnosis and management of hypertension in cats. Journal of Feline Medicine and Surgery, 19, pp. 288-303.
-
van den Broek H, et al. 2018. Prognostic importance of plasma total magnesium in a cohort of cats with azotemic chronic kidney disease. Journal of Veterinary Internal Medicine, 32, pp. 1359-1371.
1. What proportion of cats over 10 years of age are estimated to have CKD?
A. Over 10%
B. Over 30%
C. Over 50%
D. Over 60%
2. How long should clinical signs be present for in order to be attributable to CKD?
A. At least one month
B. At least two months
C. At least three months
D. At least six months
3. Changes in measurement of SDMA, USG and creatinine/urea are noted when the following percentage of nephrons are dysfunctional:
A. 40%, 66%, 75%
B. 10%, 66%, 75%
C. 30%, 40%, 60%
D. 50%, 30%, 70%
4. Target organ damage affects which organs most frequently?
A. Lungs, heart, eyes, brain
B. Heart, eyes, kidneys, brain
C. Heart, liver, kidneys, brain
D. Lungs, heart, spleen, eyes
5. What is considered an inappropriately low urine specific gravity in hydrated cats with CKD?
A. <1.030
B. <1.025
C. <1.035
D. <1.040
Answers: 1B; 2C; 3A; 4B; 5C.














