Reducing our dependence on anthelmintics for livestock
Increased frequency of anthelmintic use has been associated with the risk of resistance development. Natascha Meunier BVSc PhD DipECVPH, Programme Manager for Animal Health Ireland, describes alternate approaches to decrease the need for anthelmintic treatments
The psychologist Abraham Maslow wrote, “I suppose it is tempting, if the only tool you have is a hammer, to treat everything as if it were a nail.” Anthelmintics are not the only tool available for worm control, but they are cheap, straightforward, and until recently, highly effective tools, allowing us to easily dismiss alternatives. Control of gastro-intestinal nematodes (GIN) currently relies heavily on the use of anthelmintics; however, this approach is unsustainable over the long term as resistance is widespread and accelerating.
Ultimately, the goal of any parasite control remains the reduction or prevention of clinical or sub-clinical disease and the maintenance of production. Integrated parasite management aims to: reduce the need for treatment; use anthelmintics appropriately when needed; promote adequate refugia; and monitor for treatment effectiveness, while achieving the objective of healthy animals. All these factors are important, but this article focuses on methods that reduce the need for treatment, circumventing any potential resistance concerns.
Parasite infections differ in general from bacterial or viral infections in that multiplication requires development outside of the host. Reducing the opportunity for the host animals to ingest these infectious stages, as well as reducing the number of infectious parasites available, i.e., the pasture burden, breaks the GIN lifecycle and reduces the need for treatment. Additionally, promoting resilience and immunity in the host animals by limited exposure allows for natural defence against parasitism.
Parasite resistance is inevitable with the use of anthelmintics and is related to the frequency of their use, in that any treatments are an opportunity for selection pressure of the resistant genes. It follows then, that more frequent treatment leads to more selection pressure, and the subsequent risk of resistance development. Changes in farming practice, intensification, changes in climate, and land use have also played a role in our reliance on anthelmintic treatments.
The measures described below could go some way to reducing anthelmintic use on-farm but should be used alongside effective, timely treatment and appropriate diagnostics when required.
Grazing management and risk assessment
Pasture management, as would be expected, is primarily guided by the nutritional requirements of the animals and the practicalities of the farm. However, there are a number of general considerations that can be fairly easily adapted on farm that are effective at reducing the larval burden or avoiding high-risk pastures. Individual herds, production systems, and environments differ, so this grazing advice would need to be tailored to each farm. Additionally, some advice remains contrary to maximum grass use, such as managing grazing for longer sward height to minimise uptake of parasites which are concentrated close to the ground.
Protecting vulnerable animals, e.g., young animals, from exposure to high infection rates is something that is intuitively applied to other diseases. Similarly, weaned calves or lambs in their first grazing season should be protected by avoiding high-risk fields or pastures. The overwintering of larvae and oocysts are a risk that farmers may be unaware of, for example, favouring the same paddocks each year for newborns or groups of calves to keep them close at hand, even though these may be highly contaminated. Risk mapping involves evaluating which animals are at higher risk and matching those with fields that are likely to be at lower risk and vice versa (see Table 1). For example, a simple judgement of fields can be made considering their recent grazing history and a grazing plan adjusted accordingly. Specific risks, such as previous outbreaks of Nematodirus, lungworm, or waterlogged fields for liver fluke should be taken into account and avoided for high-risk times of year by vulnerable groups.
Co-grazing or leader/follower-type systems with older groups or mixed species can help reduce the worm burden for the other host. Older animals that have been sufficiently exposed in prior grazing seasons can be considered protected against severe disease from GIN infections and are less likely to be high egg shedders. This applies to species-adapted parasites but does not apply where hosts share a parasite or if immunity in adults is poor, for example, sheep and cattle for liver fluke.
Rotational systems have been used effectively in hot climates, where animals are returned to the same pastures after a period of time that allows for larvae to die off. In Irish conditions, it may be preferable for rotations that allow for high-risk groups to move onto low contamination pastures and graze each paddock only once in the season. If this is not possible, grazing with older animals or mowing between re-grazing with the high-risk group can reduce some of the parasite pasture burden.
For example, studies conducted in Sweden and the Netherlands found that turning out weaned, first-grazing-season calves onto low contamination pastures showed good control in the first half of the season in the absence of treatment (Thamsborg et al., 2010). Further control was maintained by moving animals either once or at monthly intervals from mid-July to low contamination pastures, although caution was still advised in the month prior to housing as a high-risk period. In Ireland, the earlier turnout, longer grazing season, and overwintering of larvae means the regular movements to low-risk pastures might be more appropriate than a single move in the season.
Delaying turnout allows for decreasing larvae that have overwintered, thereby starting the grazing season with low contamination. However, the timing of turnout is usually driven by weather conditions and available feed. An alternative is mowing before turnout which also reduces the larval burden.
Intensification results in the build-up of eggs and larvae on pasture systems during the grazing season due to high numbers of animals in limited areas. Lower stocking rates are therefore at lower risk. Extensive systems experience less worm pressure and they likely do not require frequent GIN treatments.
Pasture hygiene, or the removal of faeces, interrupts the life cycle of the worms with subsequent reduction in treatments needed. This may be viable for horse paddocks or small intensive systems such as petting zoos, although is unfeasible on larger farms.
Grazing management options ideally reduce the pasture larval burden and, by definition, also reduce the refugia on farm. It is important that the frequency of treatment should be correspondingly reduced when applying these management principles to reduce the risk of resistance development in low refugia situations.
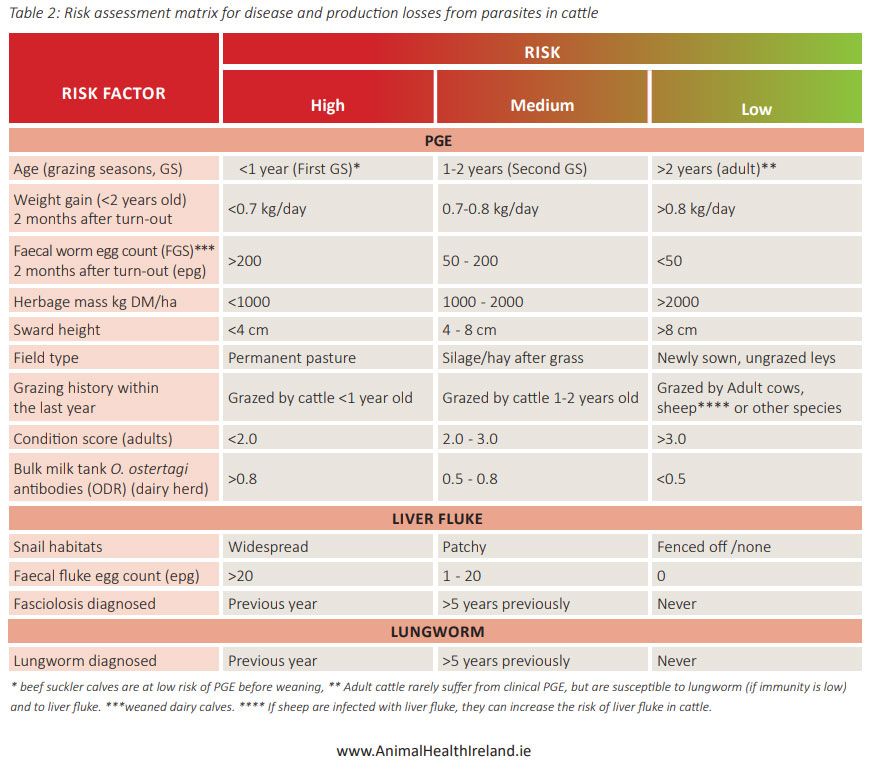
Table 1: Risk assessment matrix for disease and production losses from parasites in cattle.
Source: AHI, 2021.
Vaccination
Few vaccines are currently commercially available for helminths. In ruminants, these are for lungworm in cattle, Dictyocaulus viviparus (Bovilis Huskvac, MSD Animal Health), available in Ireland; the barber pole worm, Haemonchus contortus (Barbervax, Wormvax Australia Pty Ltd), which is available in Australia and South Africa; and for Echinococcus granulosus (Providean Hidatec EG95, Technovax) in Argentina (Vercruysse et al, 2018). While Haemonchus is present in Ireland, and cases are anecdotally increasing, it is not yet widespread and the vaccine is unlikely to be available in the near future. There is ongoing development of vaccines for other species such as Fasciola and Ostertagia.
Lungworm vaccine uptake in Ireland is limited in contrast to the perceived importance of the parasite. Over 50 per cent of cattle farmers ranked lungworm in their top three problem parasites but only two per cent of farmers reportedly vaccinated (AHI TASAH, 2023). A course of two vaccinations four weeks apart is required to be completed two weeks before turnout, which may not suit spring-born calves. Vaccination of autumn-born calves or second-grazing-season animals would be suitable on farms where lungworm is problematic.
Nutrition
When an animal or group presents with clinical symptoms such as scour, failure to thrive, or decreased milk yield, then nutritional, mineral, or energy imbalances are typically on the differential list. Animals can be overrun with parasites when additional energy stressors are placed on them and, conversely, can be resilient despite high parasite burdens when on a good plane of nutrition. Individuals suffering from a co-morbidity can also present initially for an apparent GIN complaint.
Biological control
The fungus, Duddingtonia flagrans, has been shown to entrap nematodes with a hyphal net within the dung pat thereby reducing larval load of a number of nematode species (EFSA 2020). The fungus is seeded into the dung by feeding the spores to animals in the feed ration. These fungal spores have become a commercially viable option for cattle, sheep, and horses, although it is not yet available in Europe. They have limited environmental impact on soil nematodes as the fungus largely stays associated with the faecal pat. This predatory fungus does rely on mild temperatures to grow, so can be less effective with freezing. It is not effective against pastures already burdened with larvae as it only works within the dung pat but would ideally be used to reduce contamination of ‘clean’ pastures.
Dung beetles play an important role in dung degradation and nutrient cycling which promotes grass growth. As part of this process, they have also been shown to reduce GIN larvae availability on pasture (Sands and Wall, 2017). The avermectin class of anthelmintics has been well-documented as toxic to these beneficial insects and reducing anthelmintic usage or adopting anthelmintic-sparing practices like targeted selective treatments promote their survival, in turn reducing the need for the treatments. For more information, go to the website www.dungbeetlesforfarmers.co.uk, which provides practical advice and visual descriptions of dung beetles.
Bioactive crops
Plants are widely used in traditional or folk medicine and have been extensively studied for their anti-parasitic properties, particularly in small ruminants. Plants with high levels of condensed tannins (e.g., trefoil, sainfoin, sulla) and others with secondary metabolites (e.g., chicory), as well as agro-industrial by-products, have been shown to possess anti-parasitic activity against GIN but the effect seems to depend on worm species and life stage. The variable effect is also influenced by the composition, stage, and quality of the active substances in the plant and it is not yet clear on how best to apply these on-farm.
Multi-species swards are well-researched by Teagasc, primarily for their high protein and reduced need for synthetic fertilisers, and there are current DAFM schemes encouraging their use. Some of these bioactive plant species have been included in the multi-species swards recommendations, and farmers who have adopted these should be encouraged to evaluate if they are having an effect on the parasitism in their herds. To be maximally effective, grazing of these swards should be managed to ensure all plant species are eaten, not just those that are highly palatable.
The Sustainable Parasite Control in Grazing Ruminants (SPARC) Knowledge Network is an EU-wide project that aims to reduce resistance to and reliance on anthelminants in ruminants, by identifying, applying, and sharing sustainable worm control strategies.
The SPARC project hopes to highlight the effectiveness and practicalities of implementing these types of measures on demonstration farms in the Irish farm context. If you would like to receive updates, please sign up to join with partners across Europe at: https://portal.animalhealthireland.ie/SPARC/.
Animal Health Ireland (2021), Parasite Control Technical Working Group, J O’Shaughnessy et al. A Guide to Parasite Control at Turnout. animalhealthireland.ie (accessed August 2024)
Anon. (2024) The basket of options for sustainable helminth control. COMBAR, https://www.combar-ca.eu/ Output of an EU COST action (Combatting Anthelmintic Resistance in Ruminants), unpublished.
EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP): Bampidis V et al. Safety and efficacy of BioWorma (Duddingtonia flagrans NCIMB 30336) as a feed additive for all grazing animals. EFSA J. 2020 Jul 23;18(7):e06208. doi: 10.2903/j.efsa.2020.6208. PMID: 32714465; PMCID: PMC7376537.
H. Hoste, J.F.J. Torres-Acosta, C.A. Sandoval-Castro, I. Mueller-Harvey, S. Sotiraki, H. Louvandini, S.M. Thamsborg, T.H. Terrill, Tannin containing legumes as a model for nutraceuticals against digestive parasites in livestock, Veterinary Parasitology, Volume 212, Issues 1–2, 2015, Pages 5-17, ISSN 0304-4017, https://doi.org/10.1016/j.vetpar.2015.06.026.
Sands, B. and Wall, R. (2017), Dung beetles reduce livestock gastrointestinal parasite availability on pasture. J Appl Ecol, 54: 1180-1189. https://doi.org/10.1111/1365-2664.12821.
Thamsborg SM, Roepstorff A, Nejsum P, Mejer H. Alternative approaches to control of parasites in livestock: Nordic and Baltic perspectives. Acta Vet Scand. 2010 Oct 13;52(Suppl 1):S27. doi: 10.1186/1751-0147-52-S1-S27. PMCID: PMC2994304.
Vercruysse J, Charlier J, Van Dijk J, et al. Control of helminth ruminant infections by 2030. Parasitology. 2018;145(13):1655-1664. doi:10.1017/S003118201700227X.
1. The following are factors to consider in integrated parasite management programmes:
Monitoring body condition score or average daily weight gain
FECRT
Grazing management
Weighing animals before dosing
All of the above
2. Vaccines are commercially available for which parasites?
Fasciola hepatica
Nematodirus
Dictyocaulus viviparous
Bovicola bovis
3. An example of biological control of parasites is:
Desiccation of eggs in summer conditions
Predatory fungi
Reseeding of pastures
Fencing off waterlogged areas
4. Which of the following are not needed in risk assessments for parasite control:
Evaluating the expected immunity of the hosts
Survival time of parasites in the environment
Previous grazing history of a paddock
Cost of anthelmintics or diagnostics
ANSWERS: 1E; 2C; 3B; 4D.
















