Large animal - September 2020
Equine gastric ulcer syndrome
Equine gastric ulcer syndrome is common and, therefore, should always be considered when caring for hospitalised horses as having a good understanding of this prevalent condition will allow for more effective management, writes Carly Gresak RVN BSc
Among domesticated horses, equine gastric ulcer syndrome (EGUS) is a widespread condition. Those likely to develop gastric ulcers are working horses that undergo strenuous daily exercise. According to McClure et al (2005), between 40-93% of working horses are diagnosed with EGUS. These working horses include dressage, show jumping, racing, endurance and western performance horses (McClure et al, 2005). Racehorses are reported to have the highest prevalence of EGUS, with 66-93% of individuals developing gastric ulcers during the active training period (Bertone, 2000).
Horses are ‘trickle feeders’ that typically graze for up to 18 hours per day in their natural habitat. Due to domestication, stabled horses eat at specific times when fed by their owner/keeper, usually two to three times per day. This unnatural feeding regime combined with vigorous exercise increases the likelihood of gastric ulceration, as exercise causes an increased production of gastric acid which can affect the lining of the stomach (Lane, 2015).
Veterinary professionals have an important role in helping to reduce the incidence of EGUS while the patient is under veterinary care. They also have a responsibility to advise clients on the correct care and prevention of the recurrence of gastric ulcers in patients who have previously developed them.
Stomach anatomy and gastric ulcers (types)
There are two types of gastric ulcers that horses can develop, glandular mucosal ulceration and squamous mucosal ulceration. The surface of the stomach can be either glandular or non-glandular or in the case of horses, both (see Figure 1). This means that horses have a composite stomach. The division between the glandular and non-glandular sections of the equine stomach is called the margo plicatus. The non-glandular half of the stomach is located dorsal to the cardia and includes the fundus, while the glandular region is located ventrally (Colville and Bassert, 2016).
The glandular region of the stomach is located in the pylorus and the body of the stomach. The mucosa here is dark pink in colour and contains glands that produce gastric acid. The glandular mucosa contains ducts lined by glandular cells. These cells include mucous neck cells, parietal cells and chief cells, each producing different secretions. Parietal cells produce hydrogen and chloride, mucous neck cells produce a thin mucus (which coats and protects the glandular portion of the stomach, but not the non-glandular portion) and the chief cells produce pepsinogen, which converts to pepsin in the presence of hydrochloric acid. The hydrogen and chloride secreted by the parietal cells form hydrochloric acid, which gives the stomach its low pH (1.5-7) (Collville and Bassert 2016).
The adult equine stomach can hold eight to 16 litres (L) of stomach acid and produce up to 30L of gastric secretions within 24 hours. However, the horse’s stomach is relatively small in comparison to the overall weight of the animal (Moore et al, 2001).
Figure 1: Equine gastric anatomy.
Causes of gastric ulceration
Gastric ulcers occur in horses due to high levels of hydrochloric acid, pepsin, bile acids and organic acids, which may overwhelm the protective defences of the gastric lining (such as mucus and bicarbonate). The duration of exposure to gastric acid can affect the occurrence and severity of gastric ulcers. Listed below are some examples of contributory causes.
- Strenuous exercise
Strenuous exercise may be the primary cause of gastric ulcers in horses and affects the squamous mucosal region of the stomach. The mechanical motion of galloping causes gastric acid to splash onto the squamous mucosal wall. This, on top of an inhibitory effect of exercise on gastric emptying, increases the likelihood of performance horses developing gastric ulcers in this region of the stomach (McClure, 2016). - Irregular feeding times
As outlined above, horses are grazing animals who can feed for long periods daily. When horses are housed, they may not be provided with a continuous food supply. Owing to a horse’s natural ability to graze for a large period of the day, they secrete gastric acid continuously (Andrews – ND). When horses chew, they produce saliva which acts as a buffer for gastric acid. The prolonged exposure of the mucosa to gastric acid, alongside the reduction of saliva in horses who spend less time eating, will increase the incidence of gastric ulcers. - Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) are cyclooxygenase (COX) inhibitors which can be COX-1 or COX-2 selective inhibitors. COX-1 enzymes assist in the formation of constitutive prostaglandins, which allows for various beneficial physiological activities to take place, which includes gastrointestinal mucosal protective mechanisms. By the inhibition of constitutive prostaglandins following NSAID administration, the blood flow to gastric mucosa is reduced, which decreases the production of bicarbonate and mucus (Edwards, 2019). Therefore, the inhibition of COX-1 enzymes contributes to gastric ulceration in the glandular stomach region. COX-2 enzymes cause inflammation effects and provide a pain response. By the inhibition of COX-2 enzymes, pain and inflammation are reduced which are the desired effects of NSAID therapy (Edwards, 2019). - Volatile fatty acids
Volatile fatty acids (VFAs) are a fermentation by-product of carbohydrate metabolism and can increase the likelihood of ulcers in the squamous mucosal lining. When acid concentrations are high VFAs can penetrate the mucosal wall of the stomach. VFAs may cause cellular damage, inflammation, and ulceration (Andrews – ND). Performance horses may be fed a diet high in starch (which are found in grains such as oats) to provide energy to fuel their working lifestyle, thereby potentially exacerbating the likelihood of equine gastric ulcer development (Andrews – ND). - Stress
Hospitalised horses are prone to gastric ulcers due to the high levels of stress and the possibility of periods of medically indicated feed deprivation/fasting during treatment. Stress increases the likelihood of gastric ulcer development in horses and feed deprivation will reduce the buffering effect that saliva and forage have on the squamous gastric mucosa. Food may be withheld due to gastrointestinal disease. In addition, a horse’s appetite may be suppressed if they are very ill (Row, 2008). Isolation and transportation may be other factors inducing stress in the hospitalised equine patient. Horses are social animals and being deprived of contact with other horses could induce stress, thereby increasing their likelihood of developing gastric ulcers. Similarly, transportation may increase stress levels due to the change of environment.
Clinical signs and diagnosis
The clinical signs of EGUS are generally vague and could be an indicator for many other medical issues. According to Andrews et al (2017), some clinical signs in horses diagnosed with squamous gastric ulcers include poor appetite and poor coat quality. The only definitive way of diagnosing gastric ulcers in horses is to pass a gastroscope into the stomach and visualise the gastric ulcers (Lane, 2015).
Gastric ulcers are scored using a universal scoring system. This grades the gastric ulcers from 0-4 in both the glandular region of the stomach (see Table 1) and the non-glandular (see Table 2) region. Grade 0 indicates that the epithelial wall is intact and there is no presence of hyperkeratosis or hyperaemia. Grade 4 indicates areas of deep ulceration in the epithelial wall (Boehringer Ingelheim, 2018).
Table 1: Gastric ulcer scoring system for the glandular stomach region (Boehringer Ingelheim, 2018). 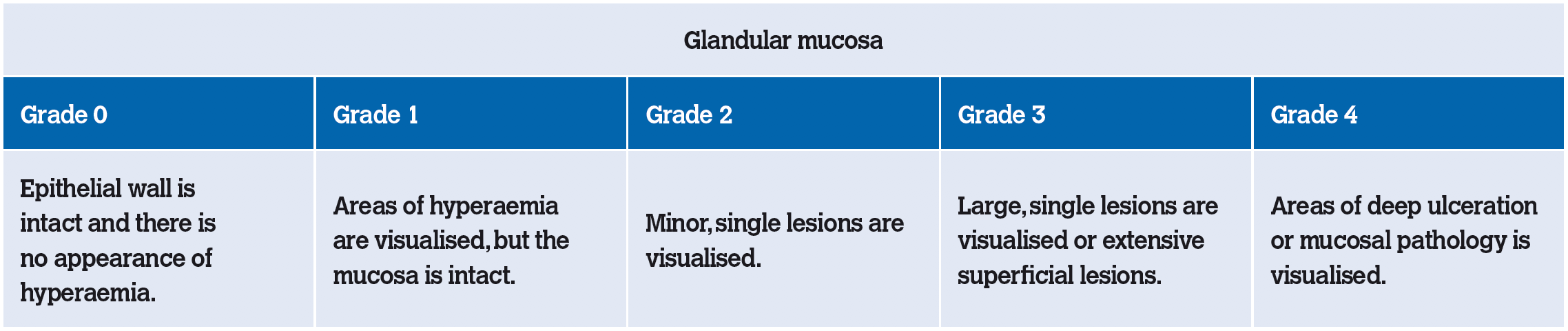
Table 2: Gastric ulcer scoring system for the squamous (non-glandular) stomach region (Boehringer Ingelheim, 2018).
Figure 2: Squamous mucosal ulceration.
Gastroscopy examination
- Patient preparation
The patient should have food withheld for 12-16 hours and water for four hours prior to a gastroscopy examination. If the patient is fasted for an insufficient time, stomach contents may hinder the visualisation of gastric ulcers and a second gastroscopy may need to be performed after an appropriate time of fasting (Lane, 2015).
The nurse should discuss fasting times with the client prior to the gastroscopy procedure. When horses are very hungry, they may ingest their bedding. To prevent the patient from eating their own bedding (such as wood shavings) a muzzle can be placed on them until ready for sedation prior to gastroscopy, or the horse can be stabled on rubber matting 16 hours before the examination is due to take place (Brazil, 2008).
Equipment
- 4-meter gastroscope
- TV screen
- Light source
- Water-based lubricant
- 60ml syringe filled with water
- Air pump
- Suction unit
- Twitch
- Lead rope and head collar
- Sedation
- Stocks
- Patient restraint and room preparation
The horse should be restrained in a stocks prior to commencing the gastroscopy procedure. This will help to ensure the safety of the horse as well as the personnel involved in the procedure. Restraint also reduces the risk of equipment being damaged by the patient. The patient should also be fitted with a head collar and lead rope. A twitch should be available to use if required while passing the gastroscope through the ventral nasal meatus (Brazil, 2008). The patient should be administered moderate levels of sedation if necessary (Brazil, 2008).
- Gastroscopy procedure
At least three people should be involved in this procedure. One person is used to restrain the patient during the procedure. Another person passes the gastroscope tube down into the stomach and the other steers the gastroscope (usually the veterinary practitioner). The veterinary nurse is usually involved with passing the tube into the stomach via the oesophagus but may perform any of these tasks.
Prior to passing the tube, the tip should be lubricated with a water-based lubricant (such as KY Jelly). This reduces the likelihood of damage to the upper gastrointestinal tract. When passing the tube, stand on the opposite side to the person restraining the horse and direct your arm over the nose of the horse and hold the opposite nostril with one hand and pass the tube with the other hand (holding the nostril allows the person passing the tube to restrain the head). Before passing the tube, rub the inside of the nostril slightly to let the horse know that you are there (Peitsmeier, 2013). Be careful not to obstruct the horse’s airway.
Insert the gastroscope tube in a ventro-medial direction, via the ventral nasal meatus, to the dorsal pharynx (the horse must swallow prior to entering the oesophagus) and into the oesophagus by directing the scope dorsal to the arytenoid cartilages of the larynx. According to Dunkel (2008), when passing through the oesophagus, the gastroscope tube may be visualised dorsal to the larynx or, at the left base of the neck, lateral to the trachea and deep to the jugular vein. The oesophagus may also be visualised on the TV screen. It is distinguished from the trachea by the absence of cartilaginous rings under the mucosa.
Once in the oesophagus, stop the passage of the gastroscope to confirm that it is correctly placed. Use the air pump to inflate the oesophageal lumen with air to facilitate passage of the tube. Pass the tube into the stomach, and through the cardiac sphincter (Lane, 2015). Once past the cardiac sphincter, the stomach should be inflated with air using the air pump and the margo plicatus should be visible (Brazil, 2008). It may be helpful to rotate the tube until the dorsal stomach appears at the top of the image, as this facilitates observer orientation. The endoscope should remain in contact with the stomach wall for the duration of the procedure (Brazil, 2008). The tube is slowly passed from the greater curvature to the lesser curvature of the stomach and to the pyloric antrum while the veterinary practitioner grades any gastric ulcers visualised within the stomach. The suction unit can be used to remove any gastric fluid that hinders visualisation of any potential gastric ulcers (Brazil, 2008). Images may also be taken and stored to record the degree of ulceration if the gastroscope possesses this function.
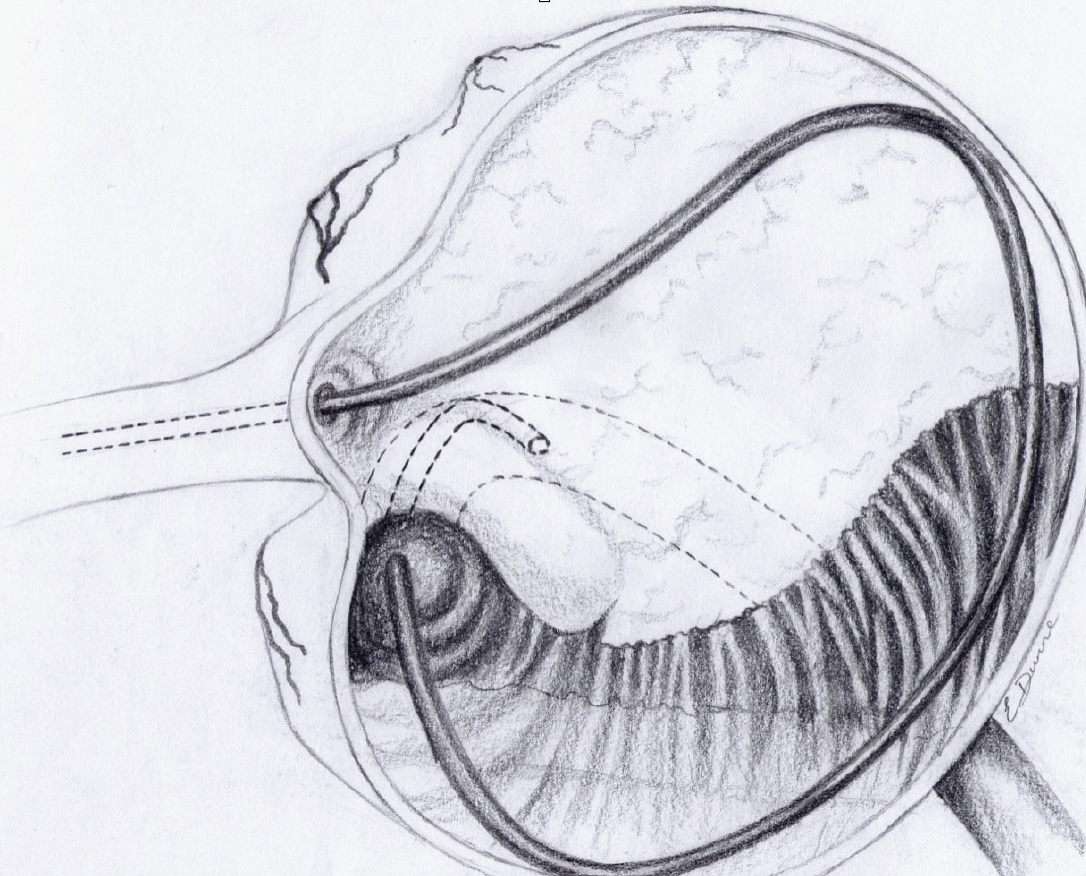
Figure 3: Gastroscopy diagram.
Cleaning and storage of the gastroscope
The gastroscope should be cleaned immediately after the procedure to prevent cross contamination by mucus drying within the lumen (Lane, 2015). Outlined below are guidelines on how to store and disinfect the gastroscope according to Meadowcroft (2019) and Boehringer Ingelheim (2018).
- Prepare a large basin and place 10L of tap water into the basin.
- Place approximately 22 grams of enzymatic powder into the basin. A sample of this cleaner should come with the scope when it is purchased or be available from the endoscope supplier.
- Clean the lumen using a biopsy channel cleaning brush.
- Remove the blue and red valve covers (blue water button and red suction button) and working channel and pass a channel cleaning brush through them.
- Replace the valve covers.
- Turn on the red suction tube to pull the water from the basin, into the gastroscope lumen.
- Once water has been pulled into the lumen of the gastroscope, press the blue button to push the water out of the scope.
- Do this three to four times.
- Remove water containing enzymatic cleaner and rinse the basin.
- Fill the basin with distilled water and repeat steps four to seven.
- Dry the gastroscope by wiping the outside of the tube with a clean towel and hang the scope to dry and store.
Administration of gastric ulcer medication
- Treatment
The most efficient gastric ulcer treatment available on the Irish market at the moment is omeprazole. Omeprazole is a proton pump inhibitor which works by reducing the secretion of gastric acid in the stomach. Omeprazole is deactivated/destroyed by stomach acid. For this reason, it is formulated as an oral paste or granules. These formulations contain pH buffers that protect the medication from immediate destruction by stomach acid. The recommended dose rate is 4mg/kg/day per os for 28 days (Lane, 2015). For the prevention of gastric ulcers, it is recommended to administer 1mg/kg/day as an ongoing treatment (Boehringer and Ingelheim, 2014). - Oral paste administration procedure
- Weigh the horse if possible, to obtain an accurate bodyweight.
- Set the plunger at an appropriate dose according to the horse’s weight (each dose division delivers a large enough dose for 100kg of body weight).
- Ensure the horse is appropriately restrained.
- Remove the syringe cap and insert the syringe into the corner of the mouth, through the diastema.
- Slowly release the contents of the syringe by depressing the plunger and spread the contents over the back of the tongue.
- Remove the syringe and tilt the horse’s head slightly to encourage them to swallow.
Omeprazole granules can be added to the horse’s feed. The feed container should be checked afterwards to ensure that the horse has ingested all the medication. The granules should only be added to dry feed and the feed should not be moistened as this may reduce the efficacy of the medication.
Prevention
Treatment with a proton pump inhibitor, such as omeprazole, can be useful in reducing equine gastric ulcer syndrome. The recommended therapeutic dose of omeprazole to treat gastric ulceration is 4mg/kg/day for approximately one month (Lane, 2015). Administering 1mg/kg/day can prevent the reoccurrence of gastric ulcers while the patient is in training (White et al, 2003).
If possible, increasing pasture turnout and a decrease in exercise intensity will help reduce the likelihood of gastric ulcers in horses; however, the lifestyle of a high-performance horse may not facilitate this (Camacho-Luna et al, 2017).
Altering feeding practices can be beneficial when treating gastric ulcers. The aim is to increase the amount of time that the horse spends chewing (Andrews et al, 2017). Avoid feeding large amounts at once and try to spread meals out by providing frequent, smaller meals during the day. Meals should ideally be offered every three to four hours during the day, rather than two to three times per day (Harris, 2001). By feeding more frequently and encouraging chewing for longer periods of time, the horse will produce increased levels of saliva which provides a buffering effect and helps neutralise stomach acid (Lane, 2015).
Some examples of commercial products that increase stimulation and prolong feeding times include alfalfa nets, ball feeders and chaff. The provision of alfalfa nets is useful as they allow the horse to chew for longer and also provide the horse with stimulation. Alfalfa nets contain small holes through which the horses must tease the forage out, therefore the speed with which they can eat is regulated. According to Andrews et al (2017), regulating feeding times will reduce the likelihood of the occurrence of gastric ulcers. Ball feeders also provide the patient with stimulation and increase feeding times (see Figure 3). The addition of chaff (chopped straw) to concentrate meals will facilitate slower eating times and promote chewing.
Providing the horse with access to a grass turn-out will reduce stress. Fresh grass will provide a buffering effect helping to reduce the incidence of EGUS (Lane 2015).
The above methods can also be provided for hospitalised horses who cannot be turned out to graze. Shellum (2008), recommends a variety of forage to be fed to sick horses to encourage feeding and therefore mimic the normal behaviour of horses. Palatable feeds can be provided to encourage horses to eat, such as fresh-cut grass, molasses, sugar beet pulp and apples. Horses should also be fed at regular intervals during the day as outlined above.
To reduce the incidence of glandular gastric ulcers, COX-2 selective NSAIDs are preferred (Richardson et al, 2017). If NSAIDs are indicated for the patient, ensure accurate doses are provided, only administer the minimum effective dose of the medication and monitor the patient closely for signs of dehydration, abdominal pain and other clinical sign of gastric ulceration which are outlined above. Dehydration will further increase the likelihood of gastric ulcerations during NSAID treatment so the patient’s hydration status should be monitored closely.
Conclusion
The high incidence of equine gastric ulcer syndrome in athletic horses requires veterinary nurses to understand why horses develop the disease and how to prevent it. This gives veterinary nurses an opportunity to enhance the welfare of hospitalised horses and provide preventative advice to owners to improve equine welfare in general. Although equine gastric ulcer syndrome is highly treatable, it is difficult to diagnose due to its vague clinical signs. Veterinary nurses can provide assistance to the veterinary practitioner in the diagnosis of the condition and implement efforts to both treat and prevent it in their patients.
-
Andrews FM. 2019. Gastric Ulcers in Horses. Merck veterinary manual [onlinehttps://www.merckvetmanual.com/digestive-system/gastrointestinal-ulcers-in-large-animals/gastric-ulcers-in-horses#resourcesInArticle [accessed 08 March 2019].
-
Andrews FM. ND. Equine gastric ulcer syndrome. American Association of Equine Practitioners [onlinehttps://aaep.org/horsehealth/equine-gastric-ulcer-syndrome [accessed 14 February 2019].
-
Andrews FM, Larson C and Harris P. 2017. Nutritional management of gastric ulceration. Equine Veterinary Education, 29(1), pp. 45-55.
-
Bertone J J. 2000. Prevalence of Gastric ulcers in elite, heavy use, western performance horses. In: 46th Annual Convention of the American Association of Equine Practitioners November 26-29, San Antonio. Lexington: AAEP, pp.256-259.
-
Boehringer Ingelheim. 2018. Equine Gastric Ulcers [onlinehttp://equinegastriculcers.co.uk/risk-factors.html [accessed 21 February 2019].
-
Boehringer Ingelheim. 2014. Gastrogard 370mg/ml oral paste [onlinehttps://www.hpra.ie/homepage/veterinary/veterinary-medicines-information/find-a-medicine/item?pano=VPA10454/058/001&t=GastroGard%20370%20mg/g%20oral%20paste [accessed 10 March 2019].
-
Brazil T. 2008. Procedures in the adult horse. In: Corley, K. and Stephen, J. eds. Equine Hospital Manual. Oxford: Blackwell Publishing Ltd., pp. 21-23.
-
Camacho-Lune P, Buchanan B, Andrews FM. 2017. Advances in Diagnostics and Treatment in Horses and Foals with gastric and duodenal ulcers. Veterinary Clinics of North America, 34(1), pp. 97-111.
- Colville T, Bassert JM eds. 2016. Clinical Anatomy and Physiology for Veterinary Technicians. 3rd ed. Missouri: Elsevier.
-
Dowling PM. 2019. Therapy of Gastrointestinal Ulcers. Merck veterinary manual [onlinehttps://www.merckvetmanual.com/pharmacology/systemic-pharmacotherapeutics-of-the-digestive-system/therapy-of-gastrointestinal-ulcers-monogastric?query=effects%20of%20bile%20salts%20on%20gastric%20mucosa [accessed 08 March 2019].
-
Dunkel B. 2008. Procedures in the adult horse. In: Corley, K. and Stephen, J., eds. The Equine Hospital Manual. Oxford: Blackwell Publishing Ltd, pp. 15-16.
-
Edwards SH. 2019. Nonsteroidal Anti-inflammatory Drugs. Merck veterinary manual [onlinehttps://www.merckvetmanual.com/pharmacology/anti-inflammatory-agents/nonsteroidal-anti-inflammatory-drugs [accessed 08 March 2019].
-
Harris PA. 2001. Nutrition. In: Coumbe, K., ed. Equine Veterinary Nursing Manual. 2nd ed. Kent: Wiley- Blackewell, pp.101-125.
-
Lane C. 2015. The veterinary nurse’s role in the diagnosis, treatment and management of equine gastric ulcers. Veterinary nursing journal, 30(11), pp. 316-318.
-
McClure SR, Murray MJ, Carithers DS, Gross SJ and Holste, J.E. (2005). Gastric Ulceration in Horses Exposed to Training and Activities Typical for Recreational Showing. In: 51st Annual Convention of the American Association of Equine Practitioners, December 3-7, Seattle. Lexington: AAEP.
-
McClure SR. 2016. Equine Gastric Ulcers: Special Care and Nutrition. American Association of Equine Practitioners [onlinehttps://aaep.org/horsehealth/equine-gastric-ulcers-special-care-and-nutrition [accessed 14 February 2019].
-
Moore JN, Melton T, Carter WC, Wright AL, Smith ML. 2001. A New Look at Equine Gastrointestinal Anatomy, Function and Selected Intestinal Displacement. In:
-
47th Annual Convention of the American Association of Equine Practitioners, San Diego, November 25-28. Lexington: AAEP, pp 53-60.
-
Rowe E. 2008. Monitoring and treating the gastrointestinal system. In: Corley, K. and Stephen, J., eds. The Equine Hospital Manual. Oxford: Blackwell Publishing Ltd, pp. 486-490.
-
Richardson L, Whitfield-Cargile C, Cohen ND, Chamoun A, Dockery H. 2017. Effect of selective versus non-selective cyclooxygenase inhibitors on gastric ulceration score and intestinal inflammation in horses. In: Proceedings of the 63rd Annual Convention of the American Association of Equine Practitioners, November 12-21 San Antonio. Lexington: AAEP, p. 377.
-
Tipperman SM. 2016. Digestive system. In: Colville, T and Bassert, J.M. eds. Clinical Anatomy and Physiology for Veterinary Technicians, 3rd ed. Missouri: Elsevier, pp. 378-415.
-
White GW, McClure S, Sifferman RL, Bernard WV et al. 2003. Prevention of Occurrence and Reoccurrence of Gastric Ulcers in Horses by Treatment with Omeprazole at 1mg/kg/day. In: Proceedings of the 49th Annual Convention of the American Association of Equine Practitioners, November 21-25, New Orleans. Lexington: AAEP.
-
Boehringer Ingelheim: Equine Health. 2018. How to clean an equine gastroscope – Equine gastroscopy expert explains the procedure [video onlinehttps://www.youtube.com/watch?v=0U6ueut3UPo [accessed 20 August 2019].
-
Meadowcroft L. 2019. Gastroscope set-up and cleaning guide by VetOvation [video onlinehttps://www.youtube.com/watch?v=hwEB5CGNQLo [accessed 15 August 2019].
-
Peitsmeier M. 2013. Equine Training: Nasogastric Intubation [video onlinehttps://www.youtube.com/watch?v=Zx14kwnESW4&feature=youtu.be [accessed 24 February 2019].
1. Which of the following is true regarding mucous neck cells?
A. Mucous neck cells produce a thin mucus which protects the glandular portion of the stomach.
B. Mucous neck cells produce a thin mucus which protects the squamous region of the stomach.
C. Mucous neck cells produce hydrogen and chloride.
D.Mucous neck cells produce pepsinogen which converts to pepsin in the presence of hydrochloric acid.
2. Which of the following regions of the equine stomach is most likely to develop gastric ulcers?
A. Glandular mucosa.
B. Non-glandular mucosa/squamous mucosa.
C. Margo plicatus.
3. What is the purpose of the red valve that is found on the gastroscope?
A. To suction liquid through the lumen of the scope.
B. To push air out of the scope and into the stomach.
4. What is the recommended dose rate of omeprazole paste to a patient prone to gastric ulcers as an ongoing treatment?
A. 3mg/kg/day.
B. 1mg/kg/day.
C. 4mg/kg/day.
5. Which of the below activities helps to prevent gastric ulceration in horses?
A. Increased pasture turnout.
B. Feeding horses small amounts of food twice a day.
C. Increased daily training periods.
D. Stress.
Answers: 1:A; 2:B; 3:A; 4:B; 5: A.
















