Focus - June 2019
Congenital Portosystemic shunts in dogs
In part one of a two-part series, Ronan A Mullins MVB DECVS, European specialist in small animal surgery and assistant professor of small animal surgery at University College Dublin, provides a comprehensive overview of congenital portosystemic shunts
What are congenital portosystemic shunts?
Canine congenital portosystemic shunts (cPSS) are abnormal vascular communications between a tributary or branch of the portal vein and a systemic vein, allowing portal blood to bypass liver sinusoids and enter directly into the systemic venous circulation.1,2 Shunting of portal blood means loss of delivery of trophic factors to the liver resulting in hepatic underdevelopment, hepatic atrophy and eventual failure, and diversion of neurotoxic substances away from the liver.
Anatomy of the portal venous system in dogs
The portal vein supplies up to 80% of the afferent blood supply to the liver, with the remainder provided by the hepatic artery.1,2 In the dog, the portal vein is made up of contributions from the caudal (draining the colon and proximal rectum) and cranial mesenteric (small intestine) veins, the splenic vein (spleen, and part of stomach via the left gastric vein) and the gastroduodenal vein (part of stomach, duodenum and pancreas). At the porta hepatis, the portal vein divides at the portal sinus into right and left branches. The right portal vein/branch supplies the caudate process of the caudate lobe and the right lateral lobe.1 The continuation of the portal vein gives off a central portal branch which supplies the right medial lobe and a smaller papillary branch which supplies the papillary process of the caudate lobe, before terminating in quadrate, left medial and left lateral branches.1 Drainage from hepatic sinusoids occurs through central veins, which eventually lead to hepatic veins that drain into the caudal vena cava.2
Classification of congenital portosystemic shunts
Congenital portosystemic shunts can be divided into intra- (IHPSS) and extrahepatic (EHPSS) morphologies. The former arise from branches of the portal vein distal to the portal sinus (right, central and left branches), while the latter arise from tributaries mentioned previously that form the portal vein (proximal to the porta hepatis). Extrahepatic shunts are more prevalent than IHPSS, with the former representing two-thirds to three quarters of all cPSS, and both are more commonly singular than multiple.1-4 Extrahepatic shunts can be broadly classified as portocaval (terminating in the caudal vena cava) and portoazygous (terminating within the thoracic cavity in the azygous vein), with portocaval most common.1-5 Specific sub-morphologies of these EHPSS have been described, including left gastrophrenic, left gastroazygous, right gastric caval, splenocaval and colocaval shunts.6,7 In a recent systematic review of published reports of 470 dogs for whom a detailed anatomic description of the morphology of their EHPSS was provided, splenic-caval, left gastric-phrenic, left gastric-azygos and shunts involving the right gastric vein were found to represent over 90% of published EHPSS.8 Intrahepatic shunts can be divided into left-, right- and central-divisional, depending on the supplying portal vein branch.1,2 Left-divisional IHPSS are the most common.9 Intrahepatic shunts typically shunt greater volumes of portal blood than EHPSS.
Aetiology of canine congenital portosystemic shunts
Extrahepatic portosystemic shunts are believed to occur due to developmental errors in utero, resulting in abnormal connections between the embryonic cardinal and vitelline systems.10 Concurrent intrahepatic portal microvascular hypoplasia, resulting in intrahepatic portal hypertension and persistent patency of vestigial blood vessels within the abdomen, has also be suggested.1 Left-divisional IHPSS are believed to represent persistence of the foetal ductus venous, a vessel which diverts blood arriving at the portal sinus from the umbilical vein to the left hepatic vein in utero.11 This vessel should normally close functionally within the first 6 days after birth (up to 9 days in Irish Wolfhounds) and structurally by three weeks.11,12 The aetiology of central- and right-divisional IHPSS is unknown, although White et al9 suggested that right-divisional IHPSS may represent a remnant of the right omphalomesenteric vein or malformation of hepatic sinusoids.
Signalment
In one study in the United States, congenital portosystemic shunts were reported in 0.18% of all dogs and 0.05% of mixed breeds.13 The majority of EHPSS are seen in small or toy breed dogs such as Yorkshire terriers, Maltese terriers, Shih tzus, and Pugs.1-4,14 Conversely, most IHPSS are found in large and giant breed dogs, including Irish Wolfhounds, Labrador and Golden Retrievers.1,2,15 A hereditary basis has been suspected or confirmed in the Yorkshire terrier, Maltese terrier and Cairn terrier.1 There is no gender predisposition in dogs.1,2 Left-divisional shunts are considered hereditary in the Irish Wolfhound (persistence of the foetal ductus venosus).16 The majority of dogs with cPSS are less than one to two years of age at presentation; however, those with portoazygous shunts are often older.1,14
Diagnosis
Diagnosis of cPSS is based on a combination of appropriate clinical signs and supporting laboratory/diagnostic imaging findings. The neurologic, gastrointestinal and urinary systems are most commonly affected.1,2,17 Dogs with portoazygous and portophrenic shunts have been suggested to demonstrate less severe clinical signs than dogs with portocaval shunts, possibly related to compression of the former by the diaphragm during breathing or the stomach after eating.1,17,18 Neurological signs are related to hepatic encephalopathy and may include abnormal behaviour, lethargy, depression, unresponsiveness, seizures, aggression, blank staring, blindness, ataxia,
head pressing, weakness, aimless wandering/pacing, disorientation, circling,
vocalising, ptyalism/hypersialism and coma.3,14,15,17,19 A variety of toxic substances have been implicated in hepatic encephalopathy in dogs, including ammonia, gamma amino butyric acid (GABA), glutamine, aromatic amino acids, short-chain fatty acids and so forth.1,2 Gastrointestinal signs may include vomiting, diarrhoea, pica, and anorexia.3,14,15,17,19 Lower urinary tract signs include stranguria, dysuria, haematuria, and pollakiuria, and are related to ammonium biurate crystalluria/cystolithiasis or bacterial cystitis.3,14,15,17,19 Polyuria and polydipsia may be related to decreased urea production by the liver and subsequent reduced renal medullary concentration gradient, increased renal blood flow, or hepatic encephalopathy (psychogenic polydipsia).2
Clinicopathologic findings
Haematology: Common abnormalities include: neutrophilia and leukocytosis, possibly related to stress response or impaired hepatic reticuloendothelial clearance of bacteria and endotoxin; and microcytic normochromic non-regenerative anaemia, possibly related to iron sequestration.1-3,14,20-22
Biochemistry: Common abnormalities include decreases in the products of the liver, including urea, glucose, cholesterol, and albumin1-3,22; decreased globulin22; and increases in hepatic enzyme activities, including alanine aminotransferase and alkaline phosphatase.1,2
Urinalysis: Abnormal findings may include hyposthenuric or isosthenuric urine due to polydipsia or decreased renal medullary concentration gradient,2 ammonium biurate crystalluria due to decreased conversion of ammonia to urea (hepatic urea cycle),1-2,14 and proteinuria, possibly related to glomerulopathy.2
Fasting ammonia and ammonium tolerance testing: Ammonia is produced in the gastrointestinal tract following bacterial degradation of nitrogenous substances.23 Increased ammonia may be observed in dogs with cPSS as a result of impaired conversion of ammonia to urea by the urea cycle in the liver.1 Elevated fasting ammonia (using a cut-off value of >46 umol/L) was found to be highly sensitive (100%) and specific (89.1%) for portosystemic shunting in one study.24 In a study by Ruland et al 2010,25 using a cut-off value of 59 umol/L, sensitivity and specificity of fasting ammonia was 85% and 86%, respectively. A transient self-resolving hyperammonemia has been described in young Irish Wolfhound pups without portosystemic shunting.26 The condition is due to a urea cycle enzyme deficiency and resolves at 3-4 months of age. Bile acid concentrations will be almost exclusively normal in such dogs.26 Dogs with cobalamin deficiency may also have hyperammonaemia.24 Ammonia tolerance testing involves measurement of fasting ammonia and 30-40 minutes after administration of 100 mg/kg ammonium chloride orally or per rectum.1,2 A reference range of < 90-100 umol/L ammonia post-challenge has been described as normal.4,27 In a recent study by van Straten et al,27 the ammonia tolerance test had a sensitivity and negative predictive value of 100% for detection of portosystemic shunting, which translates to a very low likelihood of a false negative result in a dog with a cPSS.
Pre- and post-prandial bile acids: Measurement of pre- and postprandial bile acid concentrations is probably the most commonly used test in veterinary practice to evaluate for liver dysfunction in dogs with cPSS.3,19,22,28 Reported sensitivity of combined pre- and postprandial bile acids for identification of cPSS is higher than that of fasting ammonia.23 In one study,27 sensitivity of 12-hour fasting bile acids for portosystemic shunting was 98%, with a negative predictive value of 96%. In a study by Gerritzen-Bruning et al,24 sensitivity of fasting bile acids was 92.2%. These values translate to a very low likelihood of a false negative result in dogs with cPSS. An increase in fasting bile acids in combination with an increase in fasting ammonia had a specificity of 97%, with a positive predictive value of 97%.27 In another study by Ruland et al 2010,25 using a cut-off value of 20 umol/L, sensitivity and specificity of fasting bile acids was 93% and 67%, respectively. A similarly low specificity of fasting bile acids for portosystemic shunting was identified in a study by Gerritzen-Bruning et al.24 The author recommends obtaining pre- and postprandial (+ 2 hours) bile acids for evaluation of liver dysfunction in dogs with cPSS.
Imaging findings
Abdominal ultrasound: Abdominal ultrasound is commonly performed as an initial screening test for the diagnosis of cPSS, and is usually performed conscious or under mild sedation.1 In one prospective study,29 abdominal ultrasound had a sensitivity and specificity of 95% and 98%, respectively, for detection of cPSS, with correct differentiation of IHPSS from EHPSS in 92% of dogs. Advantages of this modality include its non-invasive nature and low cost.1,2 Disadvantages include its high operator dependence and difficulty in identifying EHPSS due to their small size, variable location, and interference caused by gas filled lungs and bowel.1,2 Findings consistent with portosystemic shunting may include subjective microhepatica, identification of an anomalous vessel, decreased number of portal and hepatic veins, increased and/or variable portal flow velocity, and reduced portal vein to aortic ratio in dogs with EHPSS.1,2,29 Turbulence in blood flow in the region of the systemic insertion of the shunt may also be identified.22
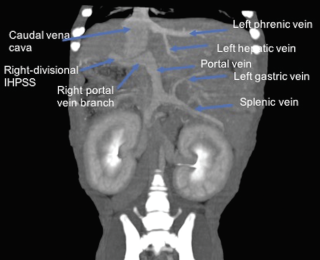
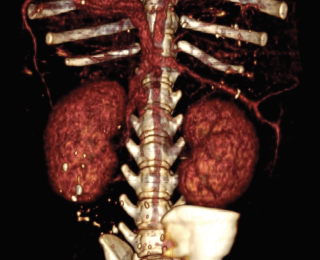
Computed tomography angiography (CTA): Computed tomography angiography has been demonstrated to be a rapid and non-invasive imaging modality for the detection and characterisation of PSS in dogs that is superior to abdominal ultrasonography.30 In a study by Kim et al,30 sensitivity and specificity of CTA for detection of PSS (96% and 89%, respectively) was significantly higher than abdominal ultrasonography (68% and 84%, respectively), with CTA almost 6 times more likely to determine the presence of a shunt than abdominal ultrasound. The use of multiplanar reconstruction (Figure 1) allows abnormal vessels to be retrospectively traced in multiple planes after the scan has been performed (unlike ultrasound). Vessels can be traced into the thoracic cavity without interference from air filled lungs.31 The ability to create three-dimensional volume-rendered shaded surface display reconstructions (Figure 2), including the ability to remove undesired arterial vessels, allows the surgeon to plan the operation more easily with excellent true-to-life display of vascular anatomy.31,32 More recently, the ability to create dynamic angiograms or cine loops permits the surgeon to dynamically observe blood flow rather than looking at a static image with traditional CTA. In a recent study by Parry and White,33 CTA demonstrated superior ability to illustrate the portal vein tributaries (cranial and caudal mesenteric, splenic and gastroduodenal vein) than intraoperative mesenteric portography.
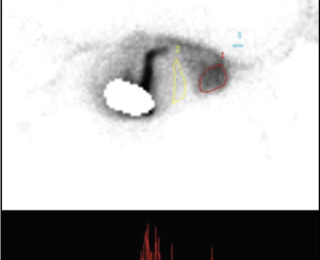
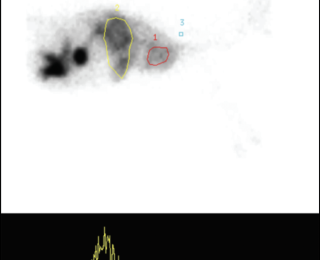
Nuclear scintigraphy: Nuclear scintigraphy can be
performed by transsplenic or transcolonic administration of radioisotope.4,22,28,31 The former involves ultrasound guided injection of radioisotope into the spleen, while the latter involves deposition of the isotope in the colon per rectum.1,2 The radioisotope technetium Tc 99M pertechnatate (99MTc pertechnatate) is most commonly used.4,22,28,31 Use of a gamma camera is required. A positive scan is defined as arrival of the radioisotope in the heart before the liver, or arrival in the heart and the liver at the same time (Figures 3 and 4). A shunt fraction can be calculated in animals with a PSS, with a ‘positive’ shunt fraction defined as ≥15% and a ‘negative’ shunt fraction as <15%.3 Most dogs with cPSS have shunt fractions >60%.2 In one study, sensitivity and specificity of transsplenic and transcolonic scintigraphy for diagnosis of cPSS was 95-100%.34 In that same study, transsplenic scintigraphy was associated with greater ability to determine the site of shunt termination (portocaval or portoazygous) than transcolonic scintigraphy. Disadvantages of this diagnostic technique include: challenges associated with differentiating single from multiple PSS and IHPSS from EHPSS; risk of radioisotope exposure to personnel; and the requirement to isolate the animal after the study. Isolation of the animal for at least 16-24 hours is required after transcolonic scintigraphy due to the six-hour t1/2 of the radioisotope, although less radioisotope is required and faster clearance of the isotope occurs with transsplenic portal scintigraphy.1
Magnetic resonance angiography: Few reports describe use of contrast MRI for detection of cPSS in dogs.35-37 Disadvantages of this imaging modality include longer image acquisition time, limited availability, and greater cost in comparison to CTA.1 In one report, image quality was described as good to excellent in all dogs.35 In another study,37 sensitivity and specificity of magnetic resonance angiography (MRA) to diagnose a shunt of any type was 80% and 100%, respectively. Correct differentiation of IHPSS from EHPSS was possible in 83% of dogs.37
Portovenography: Portovenograpy requires placement of an over-the-needle cannula most commonly in a jejunal vein at the time of coeliotomy and injection of a bolus of sterile water soluble non-ionic radiopaque contrast (eg, Iohexol) under fluoroscopic guidance.38 A mobile image intensification unit/C-arm is used to observe the portal vasculature. Portovenography is considered more invasive than other diagnostic modalities (eg, CTA, nuclear scintigraphy) as typically it requires a coeliotomy to perform. Measurement of portal pressures can be performed using the same jejunal catheter. Intraoperative mesenteric portography can also be performed immediately after temporary full attenuation to confirm that the correct vessel has been attenuated and that no further shunting vessels exist downstream.39
- Berent AC, Tobias KM. Hepatic Vascular Anomalies. In: Johnston SA, Tobias KM, eds. Veterinary Surgery: Small Animal 2nd edition. St. Louis: Elsevier Saunders. 2018; 1852-1886.
- Berent AC, Tobias KM. Portosystemic vascular anomalies. Vet Clin North Am Small Anim Pract 2009;39:513–541.
- Falls EL, Milovancev M, Hunt GB, et al. Long-term outcome after surgical ameroid ring constrictor placement for treatment of single extrahepatic portosystemic shunts in dogs. Vet Surg. 2013;42(8):951–7.
- Hunt GB, Kummeling A, Tisdall PL, et al. Outcomes of cellophane banding for congenital portosystemic shunts in 106 dogs and 5 cats. Vet Surg. 2004;33:25–31.
- Van den Bossche L, van Steenbeek FG, Favier RP, et al. Distribution of extrahepatic congenital portosystemic shunt morphology in predisposed dog breeds. BMC Vet Res. 2012;8:112.
- Nelson NC, Nelson LL. Imaging and clinical outcomes in 20 dogs treated with thin film banding for extrahepatic portosystemic shunts. Vet Surg. 2016;45:736–745.
- White RN, Parry AT, Shales C. Implications of shunt morphology for the surgical management of extrahepatic portosystemic shunts. Aust Vet J. 2018;96(11):433-441.
- White RN, Shales C, Parry AT. New perspectives on the development of extrahepatic portosystemic shunts. J Small Anim Pract. 2017;58(12):669–677.
- White RN, Burton CA, McEvoy FJ. Surgical treatment of intrahepatic portosystemic shunts in 45 dogs. Vet Rec. 1998;142:358–65.
- Payne JT, Martin RA, Constantinescu, GM. The anatomy and embryology of portosystemic shunts in dogs and cats. Semin Vet Med Surg (Small Animal). 1990;5(2):75–82.
- White RN, Burton CA. Anatomy of the patent ductus venosus in the dog. Vet Rec. 2000;146:425–429.
- Lamb CR, Burton CA. Doppler ultrasonographic assessment of closure of the ductus venosus in neonatal Irish wolfhounds. Vet Rec. 2004;155:699–701.
- Tobias and Rohrbach. Association of breed with the diagnosis of congenital portosystemic shunts in dogs: 2,400 cases (1980-2002). J Am Vet Med Assoc. 2003;223(11):1636–9.
- Mehl ML, Kyles AE, Hardie EM, et al. Evaluation of ameroid ring constrictors for treatment for single extrahepatic portosystemic shunts in dogs: 168 cases (1995-2001). J Am Vet Med Assoc. 2005;226(12):2020-30.
- Case JB, Marvel SJ, Stiles MC, et al. Outcomes of cellophane banding or percutaneous transvenous coil embolization of canine intrahepatic portosystemic shunts. Vet Surg. 2018;47(S1):O59–O66.
- van Steenbeek FG, Leegwater PA, van Sluijs FJ, et al. Evidence of inheritance of intrahepatic portosystemic shunts in Irish Wolfhounds. J Vet Intern Med. 2009;23(4):950–2.
- Kraun MB, Nelson LL, Hauptman JG, et al. Analysis of the relationship of extrahepatic portosystemic shunt morphology with clinical variables in dogs: 53 cases (2009-2012). J Am Vet Med Assoc. 2014;245(5):540–549.
- Choi SY, Lee I, Choi HJ, et al. Diagnostic imaging features of asymptomatic extrahepatic portosystemic shunt detected by CT in dogs. J Vet Clin. 2013;30:273–277.
- Hurn SD, Edwards GA. Perioperative outcomes after three different single extrahepatic portosystemic shunt attenuation techniques in dogs: partial ligation, complete ligation and ameroid constrictor placement. Aust Vet J. 2003;81(11):666–70.
- Simpson KW, Meyer DJ, Boswood A, et al. Iron status and erythrocyte volume in dogs with congenital portosystemic vascular anomalies. J Vet Intern Med. 1997;11:14–9.
- Bunch SE, Jordan HL, Sellon RK, et al. Characterization of iron status in young dogs with portosystemic shunt. Am J Vet Res. 1995;56:853–8.
- Connery NA, McAllister H, Skelly C, et al. Cellophane banding of congenital intrahepatic portosystemic shunts in two Irish wolfhounds. J Small Anim Pract. 2002;43(8):345-9.
- Bradley A. Ammonia. In: Ettinger SJ, Feldman EC, Cóté E, eds. Textbook of Veterinary Internal Medicine: 8th edition. St. Louis: Elsevier. 2017; 845–848.
- Gerritzen-Bruning MJ, van den Ingh TS, Rothuizen J. Diagnostic value of fasting plasma ammonia and bile acid concentrations in the identification of portosystemic shunting in dogs. J Vet Intern Med. 2006;20:13–19.
- Ruland K, Fischer A, Hartmann K. Sensitivity and specificity of fasting ammonia and serum bile acids in the diagnosis of portosystemic shunts in dogs and cats. Vet Clin Pathol. 2010;39(1):57–64.
- Zandvliet MM, Rothuizen J. Transient hyperammonemia due to urea cycle enzyme deficiency in Irish wolfhounds. J Vet Intern Med. 2007;21(2):215–8.
- van Straten G, Spee B, Rothuizen J, van Straten M, Favier RP. Diagnostic value of the rectal ammonia tolerance test, fasting plasma ammonia and fasting plasma bile acids for canine portosystemic shunting. Vet J. 2015 Jun;204(3):282–6.
- Adin CA, Sereda CW, Thompson MS, et al. Outcome associated with use of a percutaneously controlled hydraulic occluder for treatment of dogs with intrahepatic portosystemic shunts. J Am Vet Med Assoc. 2006;229(11):1749–55.
- Lamb CR. Ultrasonographic diagnosis of congenital portosystemic shunts in dogs: results of a prospective study. Vet Radiol Ultrasound. 1996;37:281.
- Kim SE, Giglio RF, Reese DJ, Reese SL, Bacon NJ, Ellison GW. Comparison of computed tomographic angiography and ultrasonography for the detection and characterization of portosystemic shunts in dogs. Vet Radiol Ultrasound. 2013;54(6):569-74.
- Or M, Kitshoff A, Devriendt N et al. Transdiaphragmatic approach to attenuate porto-azygos shunts inserting in the thorax. Vet Surg. 2016;45:1013–1018.
- Bertolini G, Rolla EC, Zotti A, Caldin M. Three-dimensional multislice helical computed tomography techniques for canine extra-hepatic portosystemic shunt assessment. Vet Radiol Ultrasound. 2006;47(5):439–43.
- Parry AT, White RN. Comparison of computed tomographic angiography and intraoperative mesenteric portovenography for extrahepatic portosystemic shunts. J Small Anim Pract. 2017;58(1):49–55.
- Sura PA, Tobias KM, Morandi F, et al. Comparison of 99mTcO4-trans-splenic portal scintigraphy with per-rectal portal scintigraphy for diagnosis of portosystemic shunts in dogs. Vet Surg. 2007;36:654.
- Mai W, Weisse C. Contrast-enhanced portal magnetic resonance angiography in dogs with suspected congenital portal vascular anomalies. Vet Radiol Ultrasound. 2011;52(3):284–8.
- Bruehschwein A, Foltin I, Flatz K, et al. Contrast-enhanced magnetic resonance angiography for diagnosis of portosystemic shunts in 10 dogs. Vet Radiol Ultrasound. 2010;51(2):116–21.
- Seguin B, Tobias KM, Gavin PR. Use of magnetic resonance angiography for diagnosis of portosystemic shunts in dogs. Vet Radiol Ultrasound. 1999;40(3):251–8.
- Parry AT, White RN. Post-temporary ligation intraoperative mesenteric portovenography: comparison with CT angiography for investigation of portosystemic shunts. J Small Anim Pract. 2018;59(2):106-111.
- White RN, MacDonald NJ, Burton CA. Use of intraoperative mesenteric portovenography in congenital portosystemic shunt surgery. Vet Radiol Ultrasound. 2003;44(5):514-21.