Cannabis: legislation, regulation, vet clinical use and toxicosis – an international perspective
An overview of recent research on what vets need to know about cannabis and its impact on animal health, in particular, cannabis toxicosis in pets, from an international team of veterinary writers: Nancy De Briyne and Sarah Moody, Federation of Veterinarians of Europe; Danny Holmes, Holmes Veterinary, St Anthony’s Veterinary Hospital, Caherslee, Tralee; Ian Sandler and Enid Stiles, Canadian Veterinary Medical Association, Ottawa, Canada; Dharati Szymanski, American Veterinary Medical Association, Schaumburg, Illinois, USA; Stephan Neumann, Companion Animal Clinic, Institute of Veterinary Medicine, University of Goettingen, Germany; and Arturo Anadón, Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Universidad Complutense de Madrid. This is an extract from the full article published in Animals, 2021, available at https://www.mdpi.com/2076-2615/11/3/892
As cannabis-derived products have become more available for human medical and recreational use, veterinarians are seeing more cases of cannabis toxicosis in pets. This review considers the different types of cannabis and cannabis-derived products, the existing framework of legislation and regulations for use in humans and animals as medicines and/or supplements, veterinary clinical use of these products, toxicosis, and recommendations and warnings around their veterinary use.
The use of Cannabis sp. and Cannabis-derived products is increasing globally. Concurrently, scientific interest has grown with cannabidiol (CBD)- related citations in PubMed increasing from 40 in 2000–2002 to 458 in 2014–2016. In North America and Europe, many countries have passed legislation permitting the medicinal use of certain cannabis derived products in humans, and some countries have passed laws permitting recreational use.
Although cannabinoids such as CBD may have potential for therapeutic promise, scientific evidence supporting their use in animals is currently limited and few well-controlled studies exist, most of which focus on companion animal use.
Definitions
Cannabis is an Asian herb of the family Cannabaceae—the hemp family, which has tough fibre and is often separated into a tall, loosely-branched species (Cannabis sativa) and a low-growing densely branched species (C. indica). Unfortunately, the term is used interchangeably in popular culture with marijuana although strains of cannabis can be either marijuana or hemp depending on their concentration of delta-9-trans tetrahydrocannabinol (D9-THC).
Hemp (Cannabis sativa) is legally defined in the United States (US) and European Union (EU) as any part of the cannabis plant that contains less than or equal to 0.3 per cent THC on a dry weight basis. Hemp has traditionally been farmed for industrial uses (e.g., textiles, paper, biodiesel, constructions materials), as well as for food (hemp seeds and hemp seed oil). Typically, hemp contains relatively high amounts of non-psychoactive cannabinoids. In the US, hemp is not legally recognised as a dietary supplement for either people or animals, nor as a feed supplement for animals, so products labelled as such are illegally marketed. Certain varieties of the hemp plant are legally grown in EU under license. The varieties of hemp permitted to be grown are those listed in the EU’s “Common Catalogue of Varieties of Agricultural Plant Species”. The term used to describe these varieties is “Industrial Hemp”.
Marijuana refers to a mixture of cut, dried, and ground flowers, leaves and stems of leafy green cannabis plant. The term ‘Marijuana’ is typically used for the psychoactive dried resinous flower buds and leaves of the cannabis plant (C. sativa or C. indica), but can refer to any part of the cannabis plant that contains greater than 0.3 per cent of THC.
Cannabinoids are any of the various naturally-occurring, biologically-active, chemical constituents of the cannabis plants that bind to cannabinoid receptors. Over 480 cannabinoids and other substances have been isolated.
D9-THC, more commonly called tetrahydrocannabinol (THC), is the principal psychoactive constituent of marijuana. THC is a lipid, assumed to be involved in the plant’s self-defence against insect predation, ultraviolet light, and environmental stress. The concentration of THC found in plants depends on environmental conditions, including amounts of light, moisture, soil type, pH, nutrients, and trace minerals.
Cannabidiol (CBD) is generally made from the Cannabis sativa L. plant. The plant contains hundreds of different active compounds and of these, more than 100 are cannabinoids which, depending on the compound, have either psychoactive or nonpsychoactive effects. CBD is a non-psychoactive lipid cannabinoid. CBD has been used in human medicine to mitigate anxiety, improve appetite, relieve nausea, control seizures of certain types, and assist in the management of sleep disorders.
EU regulatory framework
Human and veterinary medicinal products can be authorised in the EU either via a centralised EU system, the European Medicines Agency (EMA) or by EU national medicines agencies. There is currently no harmonised framework in the EU for the medical or recreational use of cannabis. Some form of medical cannabis is now legal in more than 22 EU countries. Several human cannabis-derived medicinal products have been authorised either via EMA or via EU national medicines agencies (see Table 1). As of February 2020, there are 17 active clinical trials that involve investigating the efficacy of cannabis and cannabinoid medications against diseases ranging from schizophrenia to endometriosis.
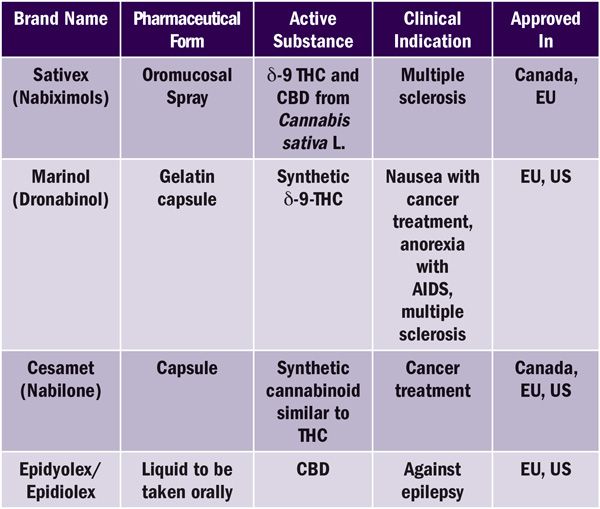
Table 1. Non-exhaustive examples of authorised cannabis-based medicines in Europe and North America at a glance.
In 2021, Luxembourg could become the first EU country to legalise recreational use of cannabis for adult use. A further 12 EU countries have decriminalised the recreational drug use of personal amounts of cannabis. In 2019, the European Parliament passed a resolution calling on the EU to implement an EU-wide policy for medical cannabis and properly-funded scientific research.
The criminal or administrative response to drug use offences is the responsibility of the EU Member States, not of the EU. According to Article 168 of the Treaty on the Functioning of the EU, “the Union shall complement the Member States’ action in reducing drugs related health damage, including information and prevention”. There are some EU laws affecting cannabis trafficking offences. The Council Resolution on cannabis of July 2004 encouraged the EU Member States to take measures against the cultivation and trafficking of cannabis within the Union and to consider taking measures against internet sites providing information on cultivation.
As per the CBD regulations of the EU, CBD infused products are not illegal in the EU unless they contain more than 0.2 per cent THC. Some EU countries, e.g.,
Belgium are stricter and have zero tolerance for THC.
Animal Feed
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) of the European Food Safety Authority (EFSA) delivered a scientific opinion on the safety of hemp (Cannabis genus) for use as animal feed. Different types of feed materials may be derived from the hemp plant: hemp seed meal/cake, hemp seed oil and whole hemp plant (including hemp hurds, fresh or dried). Further products are hemp flour (ground dried hemp leaves) and hemp protein isolate from seeds. Hemp seed and hemp seed cake could be used as feed materials for all animal species and the EFSA defined maximum incorporation rates in the complete feed per species e.g., three to seven per cent in poultry, two to five per cent in pigs for hemp seed and hemp seed cake, five per cent in ruminants for hemp seed cake and five per cent in fish for hemp seed. Feeding efficacy trials also demonstrate that hemp and its derivates may be included in diets of livestock as a good source of crude protein and essential fats.
Pet and Equine Supplements
There are many companies in the EU marketplace today selling ‘nutritional supplement’ cannabis-derived products for dogs, cats, and horses, some of which make what clearly appear to be therapeutic feed claims. These products are being promoted as aids for itching, anxiety, nausea, poor appetite, seizures, cancer, digestive problems, inflammation, immune disease, and reduced mobility due to joint pain in animals. It is against the law to make therapeutic feed claims about nutritional products. Under the EU Regulations, products for which therapeutic claims are made must firstly be approved by a National Health Product Agency or the EMA, to become a medicine and be legally manufactured and marketed.
This provides scientific data about the efficacy and safety of products. Veterinarians cannot offer scientific advice on the effectiveness of a nutritional product to treat a disease, as it is not a medicine and such claims are (a) illegal; (b) unproven; and (c) potentially unsafe. No health claims relating to hemp or CBD are authorised for use under regulation (EC) No 1924/2006 in the EU.
US regulatory framework
Human and Veterinary Medicinal Products
In the US, certain cannabis-derived substances or their synthetic analogues have been approved for use in humans. These include the most recent approval of cannabis-derived CBD, Epidiolex, for the treatment of refractory epilepsy in children. Two synthetic formulations of THC have also been approved: Nabilone, brand name Cesamet, which was approved for use as an antiemetic in patients undergoing chemotherapy; and Marinol (Dronabinol), which was approved for use as an appetite stimulant in patients with AIDS or cancer, but also used under “extra-label” guidelines as an analgesic.
More than half of US states have passed legislation permitting the medicinal use of cannabis in humans under strict guidelines. Additional states have passed laws permitting its recreational drug use. However, at this time, there are no cannabis-derived products approved by the US Food and Drug Administration (USFDA) for use in animals. Although, the aforementioned products approved by USFDA for human use can be used in animals by veterinarians under the Animal Medicinal Drug Use Clarification Act (AMDUCA) of 1994, products that have not been approved by USFDA (which includes the majority of products currently being marketed) are not available for use under AMDUCA.
Due to the considerable expense associated with pursuing USFDA approval for drugs, there is often motivation to, instead, bring such products to the market as food ingredients or food additives. There have been three hemp-derived products approved for use in human food: hemp seed oil, hemp seed protein, and de-hulled hemp seed. However, these products have been approved solely for use in human food and not for use in animal food (including pet foods and treats). At this time, no cannabis-derived products have been approved for use in food for animals. Although an application for the use of hemp seed cake and hemp seed meal as an animal feed ingredient for commercial laying hens has been submitted for review by the American Association of Feed Control Officials (AAFCO) and USFDA, it has not yet been approved.
The marketing of cannabis-derived products as supplements raises additional questions. Unlike in the EU, US regulations pertaining to “dietary supplements” for humans do not apply to products intended for use in animals. Products marketed as animal supplements are accordingly regulated as either foods or drugs (not as dietary supplements) depending on their intended use. If their intended use is therapeutic, as indicated by a therapeutic claim or the circumstances of their use, such products are regulated as drugs and must meet USFDA approval to be legally marketed; otherwise, they are regulated as foods. However, under US federal law, foods cannot contain substances that are the active ingredients in approved pharmaceuticals. US federal authorities have concluded that, because THC and CBD are active ingredients in approved drugs, they cannot be incorporated into food. Human dietary supplements also cannot contain substances that are the active ingredients in approved pharmaceuticals, including THC and/or CBD.
Accordingly, as of this writing, there are no hemp- or marijuana-derived foods, drugs, or supplements approved for use in animals in the US. Certain US states, however, have passed legislation that may impact the use of cannabis-derived products in veterinary patients. For instance, the US state of Vermont has enacted legislation defining hemp to include products intended for animals. Additionally, the US state of Nevada has enacted legislation regarding the manufacturing and marketing of hemp-derived products for animals and in California the State Board of Veterinary Medicine has established guidelines for the veterinary discussions of such products. Most recently, in the state of Michigan, legislation was approved allowing veterinarians to consult with animal owners on the use of marijuana or industrial hemp for their animals.
Use in Veterinary Medicine
At the time of writing, there are no authorised cannabis or cannabis-derived veterinary medicinal products on the market in the EU, US, or Canada. On the EU market, one product is authorised in Germany (Cardio ReVet RV4) and registered as a homeopathic veterinary medicine. In addition, one CBD product (Anibidiol) registered as a feed supplement is marketed in several EU Member States such as France and the Netherlands.
Anibidiol contains CBD and Vitamins B3 and B6. The leaflet mentions “veterinarians have good experience in using this product for behavioural problems, pain, infection, epilepsy and the consequences of tumours in dogs and cats”. The product is available without the need for a veterinary prescription.
Veterinarians could potentially use cannabis products authorised for human use ‘off-label’ in animals. The relevant legal text is detailed in Articles 10 and 11 of the EU directive 2001/82/EC, (known as “the cascade”) and the AMDUCA in the US. It is the responsibility of the veterinarian to understand their legal obligations.
Available scientific evidence pertaining to the use of cannabis products in animals is currently limited and focused on companion animals and horses. Some findings from a few well-controlled clinical studies have been published, other information is gleaned from anecdotal support, historical records, and case reports or has been extrapolated from studies related to use in humans, including the study of animal models for that purpose.
Areas of interest include use for osteoarthritis pain, other types of pain (oncologic, neuropathic), immune-mediated and inflammatory allergic disorders, cardio-vascular and respiratory conditions, and epilepsy. According to the scientific literature review, cannabinoids are mainly used in the treatment of pain, especially osteoarthritis pain.
It is difficult to estimate the real efficacy of using cannabinoids, see Table 2 showing a selection of studies investigating the effect of CBD oil on osteoarthritis in dogs.
The use of cannabinoids in the treatment of epilepsy in dogs was discussed in another clinical study. In this study, cannabinoids were given as an adjunct therapy to antiepileptic therapy for 12 dogs. Two dogs in the CBD group developed ataxia and had to be withdrawn. In the end, nine dogs in the CBD group were compared with seven in the placebo group.
Reduced seizure frequencies were observed in the CBD group, however, the proportion of dogs considered responders to treatment (50 per cent decrease in seizure activity) was similar between groups. The dogs showed no detectable analytical plasma CBD concentrations at any assessment point.
There is also interest in using CBD and other cannabis-derived products for horses, e.g., against arthritis and negative stereotypical behaviours in horses. The Fédération Equestre Internationale (FEI), the international government body for equine sports, has declared all cannabinoids as banned substances on its Equine Prohibited Substances List. Products that cannot be given to competition horses include natural and synthetic cannabinoids and other cannabimimetics.
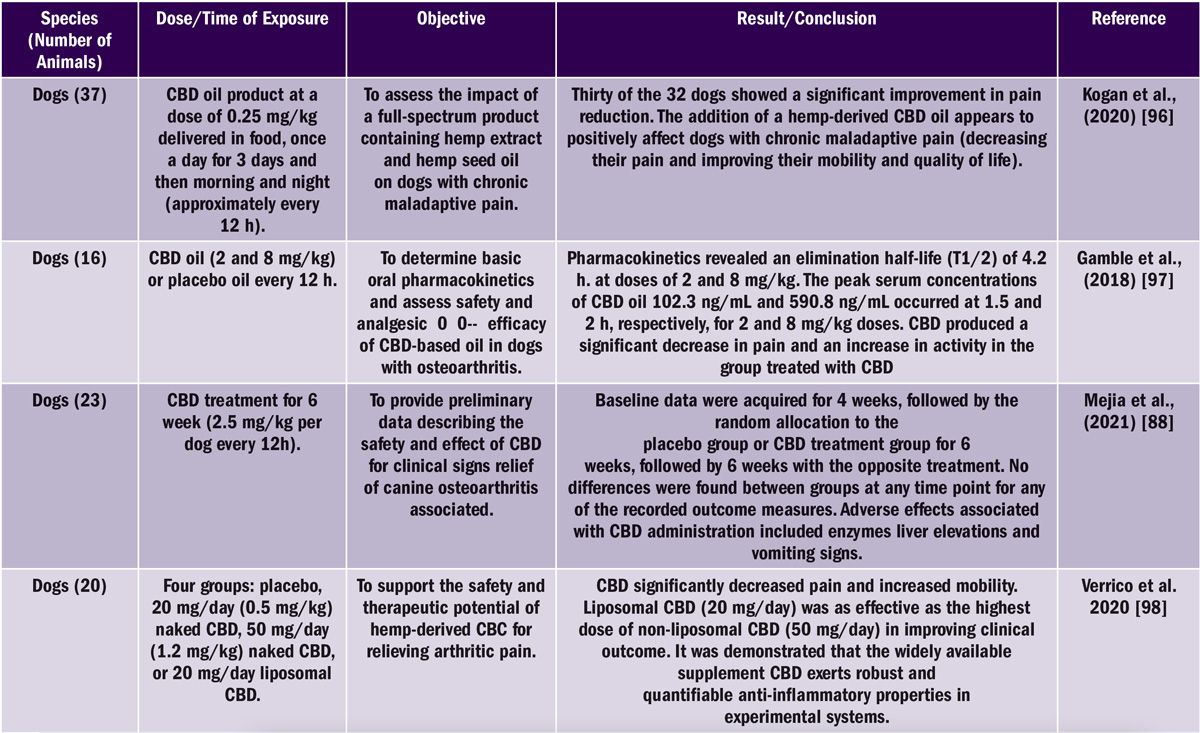
Table 2. Non-exhaustive listing of CBD studies on dogs and results.
Concerns regarding illegal product claims and unknown composition
CBD oil products may be marketed that do not conform to regulations, both animal and human. In the US, the FDA has also had to cite multiple companies illegally selling CBD products because the companies claimed they could prevent, diagnose, treat, or cure disease with some of these companies marketing products targeted toward animals.
The marketing of illegitimate products occurs particularly when the regulatory framework is not well-known and during periods of the rapid expansion of any particular sector. In the EU, all labelling information for CBD feed additives is controlled by EFSA for animal feed and human food, under the Regulation (EU) No 1169/2011, (EC) No 178/2002, and Regulation (EC) No 1924/2006. The principles behind the legislation are the 100 per cent accuracy of labelling and claims based on scientific evidence.
Veterinarians may be the most likely professional group to observe abnormal reactions, for example, psychoactive responses in animals that may have received a defective product. Veterinarians may also be approached to stock CBD products in their clinics.
In all regions, the inaccurate labelling of the identity and strength of active ingredients is of particular concern, making administration according to a dosage regimen very difficult to impossible. In Europe, only the composition of medical cannabis is known and controlled. In the US, the composition of FDA-approved therapeutic products for humans is known and controlled, but less consistency in quality assurance exists around cannabis sold for medicinal purposes by dispensaries regulated at the state and local levels. Only a few US states have extensive product tracing programmes where specific testing methods to confirm the label accuracy of the intended chemical components as well as unintended contaminants are established.
Much variation exists in which intended and unintended chemical constituents are analysed, as well as in methods of analysis. Therefore, the label accuracy of cannabinoid constituents and potential contaminants for non-FDA-approved cannabis products may vary considerably within and among US states. Few studies have been done on the safety and tolerability of these products in animals. Several studies have shown quality and safety issues related to the use of products, often illegal, labelled for animal use, some of which have been associated with toxicoses.
Cannabis toxicosis in veterinary medicine
Veterinary cases of cannabis toxicosis in dogs stem most commonly from exposure to edibles. In these cases, there may be additional toxic ingredients involved—such as chocolate, raisins, or xylitol—which result in a poorer prognosis. Cats may also directly consume the plant material.
The likelihood of pets becoming exposed is increasing as cannabis and cannabis products become more widely available and recreational drug use more commonplace. A US study in 2012 reported increased rates of toxicosis seen in dogs living in Colorado, a state in which cannabis had been recently legalised for human medicinal use; in fact, a four-fold increase in toxicoses was reported between 2005 and 2010. In 2019, the American Society for the Prevention of Cruelty to Animals’s Animal Poison Control Center noted a large jump in calls about marijuana ingestion by animals; a 765 per cent increase in the first few months over the same period the previous year. An increase was seen even in US states where cannabis has not yet been legalised. This pattern may be a result of the general shift towards cultural acceptance of marijuana use, and the growing availability and use of marijuana edibles, the leading cause of intoxication in dogs.
There is currently little research performed on thresholds for toxicosis. Although the available data suggest that CBD may be well-tolerated by animals and produces few side effects, the lack of industry-wide quality control can result in an animal’s exposure to hemp or CBD products contaminated with THC or toxins, such as heavy metals or pesticides, that may cause harm. In the US, animal poison control organisations indicate that up to 50 per cent of pets exposed to products labelled as CBD or hemp may develop clinical signs severe enough to require veterinary intervention, indicating that such products may not be pure CBD. Several deaths were reported to have been related to cannabis toxicoses, and these appear to be the result of associated complications, such as aspiration.
In dogs, excessive THC intake can easily result in clinical signs of toxicosis. Synthetic cannabinoids can have a higher potency than THC. Clinical signs of anxiety, hallucinations, seizures, psychosis, and tachycardia are reported in patients but most recover within several hours. Smaller dogs are particularly susceptible due to the smaller amount required to produce clinical signs. Cats are not immune to toxic side effects but are much more selective in their food intake and do not appear to consume cannabis edibles as often as dogs.
Cats, as a species, generally avoid eating garbage and scavenging cigarette butts, or table- or counter-surfing in comparison to dogs. They also seem to be less attracted to products with a high concentration of sugars, so we do not see them take in baked good ‘pot’ products like dogs typically do. One experimental study of cannabis exposure in cats showed that, cannabinoids caused bradycardia, hypotension and respiratory depression, which depending on the chemical composition of the product and the exposure amount, may be expected in the case of intoxication.
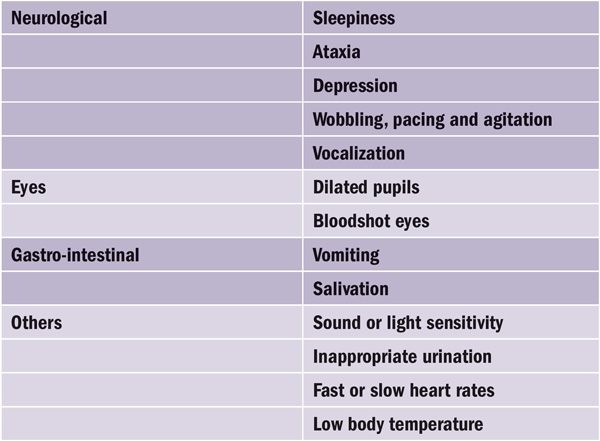
Table 3. The most common signs of excess cannabis exposure in dogs and cats.
Clinical signs
A wide range of clinical signs have been associated with cannabis toxicosis. A classic presentation is a depressed or ataxic dog that is dribbling urine. Further clinical signs are listed in Table 3. Most of them are neurological signs.
Cannabis toxicosis can look similar to intoxication with numerous other sedatives, with the most common and serious of those being anti-freeze poisoning (i.e., ethylene glycol) or ivermectin toxicosis. In most cases, the diagnosis can be made based on the
history of exposure, anamnesis and clinical signs. Cannabinoids are difficult to detect in body fluids, particularly because of their high lipid solubility and low concentrations found in urine and plasma. Urine testing can be performed but can give false negatives.
The confirmation of positive screening tests requires the use of chromatographic analytical techniques (GLC, HPLC, TLC, GC–MS) that are capable of separating and detecting the major metabolites. GC–MS is the most reliable confirmatory method, especially when used with electron impact (EI) and chemical ionisation (CI) detector modes. Common adulterants that mask a positive test for marijuana metabolites include detergents, salt, use of diuretics, and vinegar.
Treatment and prognosis
As no antidote has been described to date, the treatment of cannabis toxicosis consists of supportive care. Because of the wide margin of safety of most known cannabinoids, toxicosis is rarely fatal. Steps that should be taken include:
- If less than 30 min have passed since consumption, the animal should be decontaminated by inducing emesis and administering activated charcoal and cathartic. Repeated dosing with activated charcoal and cathartic may reduce the elimination half-life of THC by interrupting enterohepatic recirculation.
- If clinical signs have started, inducing emesis might be difficult (due to the psychoactive properties of THC) and could be dangerous if the patient is heavily sedated, as vomit could be inhaled and lead to aspiration pneumonia.
- Fluid support and keeping the patient warm may also be needed due to hypothermia. The patient should be rotated frequently to prevent dependent oedema or decubital ulceration.
- Diazepam can be given for sedation or to control seizures.
- Administer oxygen to assist respiration or relieve respiratory depression, if needed.
- Treat central nervous system depression, if needed.
- IV lipid emulsion therapy may be helpful in the treatment of severe cases.
- If the patient has lost consciousness, intense observation and support are needed. The chance of fatality is statistically small but possible.
- Recovery may take 24 to 72 hours, or longer (up to five days), depending on the ingested dose.
Conclusions and recommendations
Further research is recommended to improve our understanding of the safety and effectiveness of the use of cannabis-derived products in veterinary medicine. Current research is limited, mostly done on small samples and at times with conflicting outcomes.This suggests that cannabinoid products may potentially be beneficial in certain cases to reduce pain, particularly osteoarthritis pain, and as an adjunctive treatment of canine epilepsy.
Currently, no cannabis-derived veterinary medicinal products are authorised in the EU or North America. The off-label use of human medicinal products might be allowed to be used in animals in certain EU countries or in the US, only when using EU or USFDA-approved products, respectively. It is the responsibility of the veterinarian to understand their legal obligations.
For the future we recommend the following initiatives:
- Well-controlled clinical trials (double-blinded, placebo controlled) and pursuit of EU/North American approval or approval at the national level by manufacturers of cannabis-derived products should be conducted, so that high-quality products of known safety and efficacy can be made available for veterinarians and their patients.
- Clinical trial studies should be encouraged to investigate the potential therapeutic value and safety of hemp-derived products for companion animals.
- Harmonisation of the analytical procedure of determining the THC level in serum and oral fluids and the setting up of harmonised tolerable limits of cannabinoids in different products.
- Use of hemp-derived products for animals should require a veterinary prescription.
- Prohibition on producing pet food with cannabis-derived products without known safety and efficacy and without the knowledge of the intended purpose of the included cannabis-derived products as specified by the pet food manufacturers.
- Prohibition on producing feed supplements or beddings for food producing animals with CBD/Cannabis without known safety and efficacy and without knowledge of the intended purpose of the included cannabis-derived products as specified by manufacturers, and data on any residue in the food derived from these animals.
- We encourage veterinarians to act cautiously, as there may be risks associated with having such products in their possession if the product(s) were subsequently shown to contain illegal levels of THC. Any suspected breaches should be reported to Competent Authorities in the EU Member State where the event occurred.
Greater international cooperation is needed to help define standards, promote safety, education, research, and policy.
References available on request.
















