Is it septic? How to confidently diagnose a septic joint in the horse
David M Bolt, senior lecturer in Equine Surgery at the Royal Veterinary College Equine Referral Hospital outlines the clinical signs, diagnostic mechanisms, and management of septic arthritis in horses
Septic arthritis (SA) is commonly encountered in equine practice and represents a potentially life-threatening or career-ending emergency that requires immediate veterinary attention. Successful management of SA in adult horses includes several critical goals:
- Immediate and accurate recognition;
- Complete examination;
- Thorough timely elimination of infection;
- Reduction of inflammation and pain; and
- Return to normal function.
Joint infection in adult horses can occur after intrasynovial contamination after penetrating wounds, iatrogenic introduction after intraarticular injection or surgery and, very occasionally, hematogenous spread. Less commonly, SA can result from local extension of periarticular infection. Potentiating factors for establishing a bacterial infection include devitalised tissue, foreign material, nature and number of microorganisms involved, as well as local or systemic immunological compromise of the patient. Iatrogenic infection after intraarticular injection or surgery and penetrating wounds are the most common causes for SA in adult horses. With wounds, a variety of bacteria (gram-positive and gram-negative organisms) can be expected, whereas Staphylococcus aureus and other Staphylococci represent the most common isolates in iatrogenic septic arthritis. The size of the inoculum required to overcome synovial defense mechanisms depends mainly on the microorganism’s virulence and pathogenicity and on predisposing factors in the articular environment, such as presence of certain intraarticular medications. Several medications used in equine practice including corticosteroids and polysulphated glycosaminoglycans have been associated with a higher risk to develop SA, due to their ability to inhibit normal synovial defense mechanisms. Under normal circumstances, the synovial environment is capable of controlling large inoculations with microorganisms and of preventing their proliferation and colonisation.
Clinical signs and diagnostic methods
The main clinical signs of SA include joint effusion and variable lameness. If contamination has occurred from the outside, a wound or puncture may be visible. The degree of lameness can vary depending on size, age and type of the horse, duration of infection, as well as on pathogenicity and virulence of the infecting organism. Secondary periarticular oedema, cellulitis, or both can make identification of joint effusion difficult. Horses with open joint lacerations that are draining joint fluid are commonly not as lame as horses with closed joint infections. Mild hyperfibrinogemia and a ‘high normal’ white blood cell count (WBC) are common findings in peripheral blood analysis. However, in adult horses, the complete blood count (CBC) count and the biochemical profile are usually unremarkable.
Synovial fluid analysis
The definitive diagnosis of SA is confirmed by cytological and microbiological analysis of synovial fluid that is obtained via an aseptically performed arthrocentesis. Knowledge of the regional anatomy and different accesses to a joint cavity are important to avoid introduction of microorganisms from an overlying cellulitis. Fluid should be collected into sterile plain and ethylenediaminetetraacetic acid (EDTA) tubes. Blood culture bottles should be used for aerobic and anaerobic cultures. Ideally, a minimum of 5ml of undiluted synovial fluid aspirate should be cultured with blood culture medium to maximise the chance of isolating the infecting organism(s). In the case of an unsuccessful aspirate or in joints with a small volume (eg. the distal tarsal joints), it may be necessary to first infuse the joint with sterile isotonic saline prior to obtaining an aspirate. Urea concentration in synovial fluid of joints closely mirrors the urea concentration in serum. This can, therefore, be used to accurately calculate the dilutation that occurs with this technique.
A cloudy or turbid appearance of synovial fluid is strongly suggestive of infection. The viscosity of synovial fluid is directly related to the hyaluronan content which decreases with SA. Mucinous precipitate quality (MPQ) or mucin clotting is a semi-quantitative measure of hyaluronan concentration and is poor synovial fluid of joints with SA. Practically, viscosity can be estimated by watching the synovial fluid drop from a syringe or by separating a drop between the examiner’s thumb and index finger.
Normal synovial fluid protein is usually less than or equal to 2g/dL. Total protein concentration increases with SA and often rises above 4g/dL, although this varies and may often be lower earlier in the disease process. Normal synovial fluid has fewer than 200 cells per µl (less than 10% of neutrophils). Synovial fluid from infected joints can have a total WBC count (WBC) of more than 50,000cells/µl (predominantly neutrophils). Sequestration of inflammatory cells in intraarticular fibrinocellular conglomerates (pannus) can occur. Microorganisms can be directly identified on cytological smears in approximately 25% of cases.
Synovial biopsy
Biopsy of the synovial membrane can be performed during arthroscopic exploration and lavage of a joint. Although histology does not always allow for a clear distinction between infectious and non-infectious inflammation, co-culturing synovial biopsies and synovial fluid has been reported to increase the chance of obtaining a positive culture result in horses with SA and help with antibiotic choice. This technique is rarely used in equine practice.
Diagnostic imaging
Radiography
Diagnostic imaging is an essential part of the work-up of horses with suspected SA. Plain radiographs should first be obtained in order to evaluate for presence and extent of bone pathology. Bony involvement can include osteitis, osteomyelitis, physitis, osteoarthritis or fractures that communicate with or are related to the affected joint. Contrast radiography such as fistulograms or positive contrast arthrograms (see Figure 1) can be used to confirm communication of a wound with a neighboring joint or can help identifying cartilage damage that is not detected on routine radiographs.
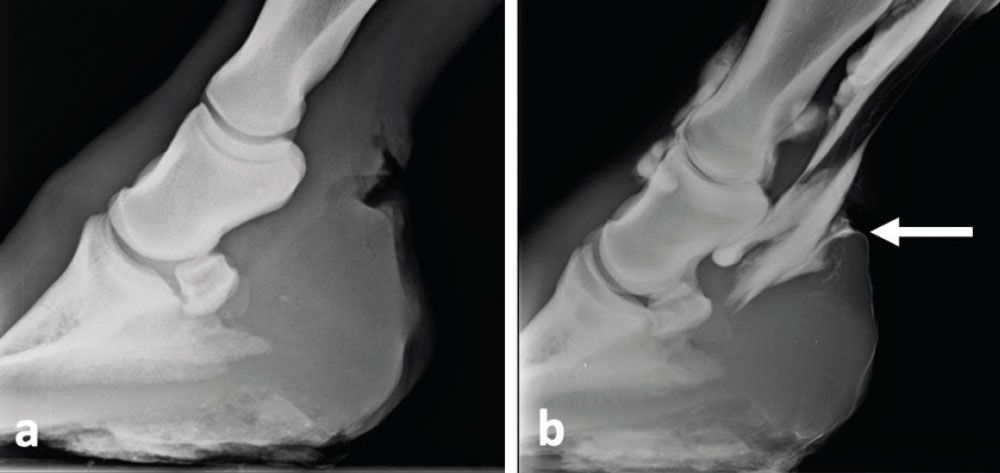
Figure 1: Radiographic study of a horse presented with a fresh laceration in the pastern area: A) plain radiograph; B) positive contrast tenogram. The digital flexor tendon sheath has been distended with iohexol and physiologic saline. The radiographic contrast material can be seen leaking from the synovial structure through the wound (arrow).
Ultrasonography
Ultrasonography is very useful in the diagnosis of SA. The technique can be used for identifying communication between wounds and adjacent joints, to determine the degree of effusion in the affected joint, to assess the nature of the synovial fluid, to subjectively identify synovial inflammation, to evaluate integrity of parts of the articular cartilage and to identify foreign bodies that are not readily identified on plain or contrast radiographs. In addition, ultrasonography can be used for guiding arthrocentesis during collection of synovial fluid for analysis. Ultrasonography can be difficult or impossible with fresh wounds where there is a large amount of subcutaneous air accumulation.
Advanced imaging modalities
Radionucleotide imaging is used extensively in human medicine to differentiate between septic and aseptic osteoarticular disease. Although radionucleotide imaging is not routinely used in equine practice, 99mTc-labelled leukocytes have been reported to help in the diagnosis of occult infections. Computed tomography (CT) and magnetic resonance imaging (MRI) are becoming increasingly available and affordable in the diagnosis of musculoskeletal disease in horses. Although plain and contrast CT and MRI studies can provide superior detail of bone and soft tissues over radiography, cost and the necessity to anaesthetise the animal for the majority examination of the appendicular skeleton in most referral centers preclude their routine use for the diagnosis of SA. On the other hand, standing-low field MR examination of the distal limb can provide very valuable additional information, particularly with solar penetrations.
Conclusion
Synovial fluid analysis remains the gold standard to confirm infection of joints, tendon sheaths and bursa. A rapid diagnosis is important in order to timely initiate the correct, targeted treatment, such as endoscopic lavage. This is particularly important with wounds in the vicinity of synovial structures. Unfortunately, it is not always possible to aseptically obtain a synovial fluid sample for laboratory analysis. Additional techniques, such as infusion and aspiration of sterile physiologic saline, as well as diagnostic imaging modalities, such as positive contrast radiography, ultrasonography and occasionally advanced imaging modalities can be helpful in these situations.
Recommended reading
Bryant HA, Dixon JJ, Weller R & Bolt DM. 2019. Use of Positive Contrast Radiography to Identify Synovial Involvement in Horses with Traumatic Limb Wounds. Equine Vet J 51: 20-23.
Lugo J, Gaughn EM. 2006. Septic arthritis, tenosynovitis, and infections of hoof structures. Vet Clin N Am Equine Pract 22: 363-388.
Morton A. 2005. Diagnosis and treatment of septic arthritis. Vet Clin N Am Equine Pract 21: 627-649.
This article is also available on page 194 of the 2019 London Vet Show Proceedings.















