The cost of not vaccinating: a case study
Boehringer Ingelheim Animal Health’s 2020 BVDzero case study award scheme culminated recently with two virtual online sessions in which the authors of the top 10 studies presented their cases. Paul Crawford, a self-employed veterinary consultant and farmer based in Larne, presented a bovine viral diarrhoea case report of incursion into a naïve herd in Northern Ireland, and we present the details of his report here
Bovine viral diarrhoea (BVD) is a worldwide disease of cattle that is of economic and welfare significance. It can be controlled and has been eradicated in several countries already. The virus generally causes a short-term infection. Clinical signs include fever, respiratory signs, drop in milk production, diarrhoea, embryonic death, abortion and occasionally death. These animals are only transiently infected (TI), as they recover from the infection when virus shedding stops. However, infection during the first third of pregnancy results in the unborn calf becoming persistently infected (PI) as the calf's immune system will fail to recognise the virus as something foreign and the virus remains active in the calf throughout its life. The PI calf's immune function is damaged permanently leaving it more susceptible other diseases eg. pneumonia. Most PIs fail to thrive and will die between six and 24 months. They either succumb to other disease or the BVD virus changes (mutates) and the animal develops mucosal disease. This is invariably fatal. PIs' continual shedding of virus is the main source of infection to other cattle.
Many studies have indicated a positive cost benefit analysis favouring control and eradication of BVD. Control of the disease instead relies on a combination of identification and euthanasia of PIs and vaccination. The main risks for BVD entering a herd are the movement of livestock (including boundary contact), personnel and equipment.
History and background
This coastal family farm milked approximately 250 mainly Holstein Friesian cows. A block calving pattern starts in August for six months. Cows are housed through the milking period. Some cows graze during their dry period when possible. All calves are retained and reared as dairy replacements or for beef.
The housing for adult dairy stock, replacement heifers and the main calf house as well as the parlour, cattle footbath and crush facilities are under one continuous roof. This, effectively, creates one airspace with common walkways and collecting yards.
One block of land surrounds the dairy unit while further blocks spread over a seven-mile radius are used for grazing and silage production. There is a total of 672 acres. Few boundary fences meet the recommended 3m buffer width. Approximately 25 herds graze fields adjacent to one or more farm boundary.
Dairy replacement heifers were summer grazed in their first and second season away from the main unit. In September (2016) as they approach 14 months of age, they returned to the dairy unit to be observed for bulling and subsequent artificial insemination (AI). A sweeper bull was used from early 2017 onwards. Family, full time, part time and occasional staff are employed.
Previous BVD testing on the farm
The herd was enrolled on the Northern Ireland BVD eradication scheme prior to the 2016 calving season. This was the first year of testing on the farm and all calves tested negative. Discussion was held between the farmer and his regular vet around vaccination, the farmer did not perceive that he had a problem and vaccination was declined. In August 2017, calving started and a batch of 79 tags were submitted for testing in late August.
Twenty-one of these samples came back positive and 58 were negative. The farmer elected to retest 14. All retests were positive.
In September and November, a further 25 animals and a single animal tested positive (respectively) by tag testing. A total of 47 PIs were identified and during the same period 104 calves tested negative.
The 47 calves were culled directly because of a positive test; 20 of these were female and the majority of those would have been dairy-herd replacements.
Additional mortality arose during the period when PIs were on the farm because of an increase in the number and severity of cases of respiratory disease. Fatal cases of suspected acute BVD were seen in test-negative calves. This included one calf with haemorrhagic syndrome, tongue and oral ulceration.

Figure 1: One PI (yellow arrow) was missed and only slaughtered in February 2019 when, due to poor growth, it was retested and proven blood ELISA positive. Pen mates are one year younger.
Analysis of the results
Given the number of PIs on the farm at the time, it had to be assumed that a virus would be circulating in the milking herd and bulling heifers. As the breeding season was about to start when the first positive results came through, an immediate decision was made to vaccinate all at-risk females. The availability of a single-dose vaccine with rapid onset of immunity was ideal in this situation.
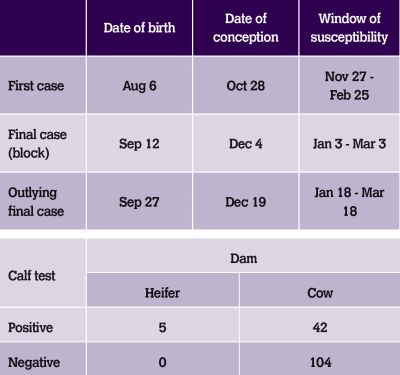
Table 1.
Heifers were significantly under-represented in the pool of PI dams. No calves born to heifers were PI after August 20. Were the rest of the heifers already immune? Was this the source? The source of infection risks identified were categorised as to their relative risk of being the source of BVD introduction based on the dates infection was thought to be circulating in the breeding herd
Risk of introduction RATIONALE
Both foot trimmer and vet were observed to maintain good levels of cleanliness and hygiene. However, their direct contact with multiple animals means any small biosecurity break would have a high chance of onward transmission.
While visitors to the farm often came direct from their own farms, they did not often have direct physical contact with livestock; however, occasional direct and frequent indirect contact could occur. The silage-contracting vehicles, meal and milk lorries never had direct contact with livestock though would drive through areas in which cattle may be walked through, and were washed with aerosol and run off heading into animal housing. Returning livestock seem the most likely source of infection.
Cost of outbreak
Business cost
- One full line of heifers was lost from the parlour. In addition, they would have calved prior to entering the milking herd. It is also likely that around the time of infection of the pregnant cows, several of these will have suffered early embryonic death or aborted.
- Currently the surviving heifers are yielding on average 34L/day meaning a loss of production of £174 a day. The profit margin from the 28 beef animals was also lost from the business and several thousand pounds for calf death and morbidity around the time of the outbreak.
Emotional cost
- Speaking about the outbreak, the farmer said: ‘‘When I started getting all these results back, I was totally devastated. What was going to happen? Was the whole herd going to be affected? It was a very worrying and stressful time. It took an emotional toll. It was a lot of stress on the family and on family life. Lots of anxiety and worry.”
- His wife added: “For someone not as mentally well as him, it [the results] could have had a detrimental effect. He had the emotional support of his family, but someone who is a lone farmer could have suffered even more. Farmers don’t talk about their emotions, but they should. There should be more support for them.’’
While the economic costs of the disease have been well documented there is less literature available on the human/emotional cost of an outbreak. The nature of the testing process means a drip feed of anticipation and bad news over an extended period of time. Extensive social effects research has been published in the wake of the 2001 Foot and Mouth outbreak. When an outbreak of this magnitude occurs on a family farm it creates stress and worry. In the face of extensive outbreaks, the testing authorities should consider opportunities to offer support to farm families or direct them to local support services.
Discussion and conclusion
Vaccination of the herd could have prevented the outbreak, financial losses and emotional toll. As we head into a world that is progressively being cleared of BVD, we must never lose sight of the importance of vaccination to protect herds, regions and countries where the disease has been successfully eradicated. The cost in animal welfare, farmer mental health and faith in disease-control programmes is far too high to leave a naïve population exposed to reintroduction from rogue PIs or trans-boundary, accidental spread by man, beast or machine.
Acknowledgement
This report and images are republished courtesy of Boehringer Ingelheim. The BVDzero Awards aim to promote the proactive investigation and management of both clinical and subclinical BVD in cattle herds. Presentations from all the BVDzero case study award finalists can be viewed on www.youtube.com/c/BVDzero
-
Bexton S. Hedgehogs. In BSAVA Manual of Wildlife Casualties. 2nd edn. 2016. Eds E. Mullineaux, E. Keeble. BSAVA. pp 117-136.
-
Bexton S, Couper D. Veterinary care of free-living hedgehogs. In Practice 2019; 41(9):420-32.
-
Bexton S, Nelson H. Comparison of two systemic antifungal agents, itraconazole and terbinafine, for the treatment of dermatophytosis in European hedgehogs (Erinaceus europaeus). Vet Dermatol. 2016; 27(6): 500-504.
-
Bunnell T. The Assessment of British Hedgehog (Erinaceus europaeus) Casualties on Arrival and Determination of Optimum Release Weights Using a New Index. Journal of Wildlife Rehabilitation 2002; 25(4): 11-21.
-
Chaprazov, T., Dimitrov, R., Stamatova Yovcheva, K. & Uzunova, K. Oral and dental disorders in pet hedgehogs. Turkish Journal of Veterinary and Animal Sciences. 2014. 38, 1-6.
-
Lawson B., Franklinos, L. H. V., et al. Salmonella Enteritidis ST183: emerging and endemic biotypes affecting western European hedgehogs (Erinaceus europaeus) and people in Great Britain. Scientific Reports. 2018. 8, 1-11
-
Miller EA, ed. Minimum Standards for Wildlife Rehabilitation. 4th edn. 2012. National Wildlife Rehabilitators Association and International Wildlife Rehabilitation Council, St Cloud, MN.
-
Molony SE, Dowding CV, Baker PJ, Cuthill IC et al. The effect of translocation and temporary captivity on wildlife rehabilitation success: an experimental study using European hedgehogs (Erinaceus europaeus). 2006. Biological Conservation 130, 530-537.
-
Robinson, I. Hedgehogs. In Hand-Rearing Wild and Domestic Mammals. Ed L. J. Gage. Iowa State University Press. 2002. pp 75-80.
-
Yarnell RW, Surgery J, Grogan A et al. Should rehabilitated hedgehogs be released in winter? A comparison of survival, nest use and weight change in wild and rescued animals. Eur J Wildl Res 65, 6. 2019.
1. When is it appropriate to see a hedgehog during the day?
A. During the summer months
B. Nursing or pregnant females
C. Young juveniles
D. It is normal for hedgehogs to forage for food during daylight hours
2. What weight should a captive hedgehog reach before releasing them into the wild?
A. >650g
B. >450g
C. >550g
D. >1000g
3. What drug is considered the treatment of choice for Lungworm infection in hedgehogs?
A. Fenbendazole
B. Injectable ivermectin
C. Toltrazuril
D. Levamisole
4. Which of these agents are not of zoonotic risk when handling hedgehogs?
A. Trichophyton erinaceid
B. Salmonella Enteritidis PT11
C. Crenosoma striatum
D. Capillaria aerophila
5. What is the main concern regarding feeding a purely wet food diet to captive hedgehogs?
A. Obesity
B. Nutritional imbalances
C. Dental tartar
D. Anorexia
6. When is it appropriate to keep a wild hedgehog as a pet?
a. If they bond with you while in captivity
B. If they are close to the end of their natural life
C. If they are permanently disabled and cannot be released into the wild
D. It is never appropriate to keep a wild hedgehog as a pet















