Companion animal - October 2019
The prevalence of intestinal parasites in dogs and cats in the greater Dublin area
Parasites of the intestinal tracts of dogs and cats are important from both clinical and public health perspectives. However, in recent years, there has been little research on the prevalence of intestinal parasites in Ireland.1 This encouraged University College Dubliln veterinary student, Allison Mangan, to carry out a summer research project aimed at investigating the prevalence of intestinal parasites in dogs and cats
Studies from elsewhere in Europe indicate that Trichuris vulpis, Toxocara canis, Toxocara cati, Cryptosporidium parvum, and Giardia duodenalis are among the most commonly isolated canine and feline intestinal parasites.2 The aims of this study were: to determine the prevalence of intestinal parasites in dogs and cats in Ireland; to determine the popularity and frequency of use of anthelmintic products available on the market; and to determine the accuracy of microscopic analysis in comparison with the Crypto-Giardia FASTest™.
Materials and methods
From mid-June until the end of July 2018, fresh faecal samples were collected by participating dog and cat owners and local animal welfare shelters immediately post-defecation. Owners were advised to store samples at 4°C until they were delivered to the Veterinary Parasitology Laboratory at University College Dublin (UCD). Owners were also asked to provide the following information with the sample: species, breed, age, gender, date of last anthelmintic treatment, and anthelmintic product(s) used. On arrival at the laboratory, a small amount (approximately 1.5g) of each faecal sample was stored in an Eppendorf tube and frozen at -20°C until required. The remainder of the sample was stored at 4°C and analysed within 24 hours of receipt. Routine faecal analysis was carried out to determine the presence of nematode eggs, Cystoisospora spp. and Cryptosporidium spp. oocysts, Giardia spp. cysts, and Angiostongylus vasorum L3 larvae.3 Frozen samples were thawed to room temperature (20°C) prior to analysis using the Crypto-Giardia FASTest™ (Megacor, Austria). Animals were categorised as being either under one year of age or over one year of age. The research was approved by the Animal Research Ethics Committee of University College Dublin (Ireland); (AREC-E-18-36-Aungier).
Results and discussion
A total of 167 animals were sampled, of which 10 were under one year of age; 109 were over one year of age; five were cats less than one year of age; and 43 were over one year. Owners reported the time of last anthelmintic treatment, as summarised in Table 1. The time of their last anthelmintic treatment was unknown for 59.3% (99/167) of animals mainly because these animals were sourced from rescue centres. From Table 1, it can be seen that of the 68 animals with a known history of frequency of anthelmintic treatment, all were dogs and only 44% (30/68) were dewormed frequently (≤2 months).2
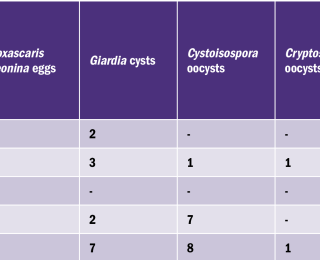
The intestinal parasites detected during laboratory analysis are summarised in Table 2. Toxocara spp. were the most commonly isolated nematode (6%; 10/167). One kitten and five adult cats were found positive for Toxocara cati, yet only the kitten showed clinical signs of diarrhoea. Toxocara spp. are of particular interest due to the risk of zoonosis. In humans, Toxocara larvae may undergo somatic migration, causing serious health concerns if the larvae migrate to the eyes, nerve tracts, or within the brain.4 Ocular toxocariosis may result in retinal damage and vision loss, while visceral toxocariasis may lead to fever, fatigue, and abdominal pain. Young children are at the highest risk of becoming infected.5 The European Scientific Counsel Companion Animal Parasites (ESCCAP) guidelines6 recommends the following:
- Dogs, that are free to roam unsupervised, should be treated with an anthelmintic 12 times per year against roundworms and tapeworms, or should have a faecal examination carried out;
- Cats that are free to roam outdoors, should be treated at least four times per year or have faecal analysis performed at least four times per year;
- Dogs and cats, sharing homes with young children or immunocompromised individuals, should be treated 12 times per year if excretion of worms is to be excluded;
- Dogs who are not free to roam unsupervised and are not in contact with other dogs outside the household should be treated one to two times per year against roundworms and tapeworms, or be treated based on faecal examination;
- For cats that live indoors, they should either be treated against roundworms or have faecal examinations carried out one to two times per year. If the risk factor cannot be judged clearly, the animal should have a faecal examination or be dewormed four times per year; and
- Deworming one to three times per year does not provide sufficient protection.6
Vets should inform their clients about the existence of ESCCAP and similar guidelines, as a way to further educate them and as an explanation as to why they are advising their practice protocol for worming. There were no cases of Angiostrongylus vasorum, Trichuris vulpis, or Uncinaria stenocephala or cestode proglottid segments identified on examination. The most commonly isolated protozoa were Cystoisospora spp. oocysts (4.8%; 8/167), followed by Giardia spp. (4.2%; 7/167). Cryptosporidium oocysts were isolated from one adult dog. Out of 10 pups tested, two were positive for Giardia, one of which showed diarrhoeic clinical signs. Despite conflicting evidence, some research in dogs and cats has shown that both Giardia spp. of genotypes A and B and Cryptosporidium spp. are potentially zoonotic.7,8,9 One pup, one adult dog, and one adult cat, who were showing clinical signs of diarrhoea, were positive for Giardia. In relation to treatment, Panacur™ is the only product which is licensed by Health Products Regulatory Authority (HPRA) in Ireland as an aid in the control of Giardia in dogs, but not in cats.10 No products are available to treat Cryptosporidium or Cystoisospora infection in dogs and cats. This fact highlights the importance of carrying out faecal analysis to ensure correct endoparasite control. For parasites whose eggs or larvae are passed in the faeces, the control of parasite stages in the environment is essential to minimise the infection risk to other animals or humans.6 Suggested methods include the rapid disposal of animal faeces, which, in Ireland, can legally be disposed of with household waste. Dog owners are legally required under Section 22 of the Litter Pollution Act 1997 to remove their dog’s faeces and dispose of it properly. Other methods include covering of sandpits, or regular replacement of uncovered sand. Direct sunlight and UV light can also aid in controlling environmental contamination, as it contributes to desiccation of endoparasite eggs.
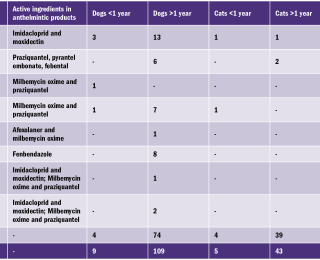
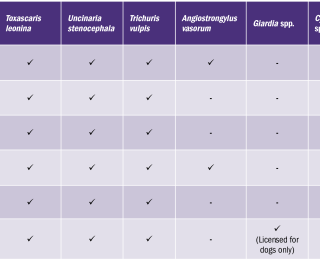
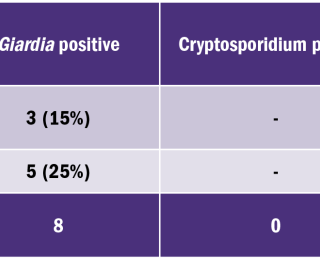
The anthelmintic products used by participating owners are summarised in Table 3. The most popular anthelmintic product was the spot-on product, Advocate™ which was used in 37.5% (18/48) of animals. In 6.25% (3/48) of cases, Advocate™ was used in combination with a deworming tablet containing milbemycin oxime and praziquantel. The remaining 56.5% (27/48) used one of the tablets listed in Table 3. The target species of the anthelmintic products used by owners in this study are outlined in Table 4. No products were effective in treating or preventing infection with Cystoisospora or Cryptosporidium spp. It is interesting to note that, despite all of the anthelmintics mentioned being effective against Toxocara spp., it is the most prevalent endoparasite identified in the survey (Table 2). Hence the need for owners to be vigilant regarding regular deworming. Twenty random samples were analysed using the Crypto-Giardia FASTest™. The results of this test were compared with microscopic findings, as summarised in Table 5. Of the 20 samples analysed using the Crypto-Giaridia FASTest™, five were identified as positive for Giardia, while only three of those samples were identified as positive using microscopy. Both Cryptosporidium and Giardia are shed intermittently, meaning they will not always be detected with light microscopy alone. The Crypto-Giardia FASTest™, is particularly useful in identifying animals who are asymptomatic. These animals pose a threat by acting as a reservoir of infection for other susceptible (young or immunocompromised) animals. In discussion with owners participating in this study, more than 50% were worming less frequently than recommended by anthelmintic data sheets (Table 1). Owner comments ranged from ‘I forgot to worm’ to ‘I only wormed the animal when I first got it’. Further questioning identified factors such as ‘cost’ and ‘unwillingness to treat without clinical evidence of infestation’. This apparent ‘resistance’ to regular worming requires further investigation due to the zoonotic risk of some endoparasites. Potentially zoonotic parasites identified in this study include Toxocara spp., Cryptosporidium spp., and Giardia spp. The study identified that Advocate™ was the most popular anthelmintic used, with 31% (18/48) of owners using this spot-on product, due to ease of administration (Table 3). Only Toxocara canis/cati and Toxascaris leonina were identified in the samples examined (Table 2). Three protozoan species were identified; Cystoisospora spp., Giardia spp., and Cryptosporidium spp. The prevalence of intestinal parasites found in this study differ from reports elsewhere in Europe. This study identified: Toxocara species as the most prevalent nematode (6%, 10/167), while another study found that Trichuris vulpis was the most commonly identified nematode (3.8%).2 Both studies are in agreement that the potentially zoonotic protozoan, Giardia, was the most significant protozoan seen; with 4.2% (7/167) of cases identified in the present study and 6.3% identified by Hinney et al., in 2017.2 Of the anthelmintic products used in this survey, only Panacur™ has proven efficacy against Giardia. The recommended dose is 50mg/kg BW/day on three successive days followed by faecal re-examination to determine if a further course is required. Giardia accounts for 26% (7/27) of the positive cases identified in this study (Table 2). Based on this information, it is recommended that faecal analysis be carried out routinely in order to determine which anthelmintic product, or combination of products, would be most efficacious in individual cases. Use of anthelmintics based on faecal analysis results would be an important step in the prevention of anthelmintic resistance.
The Crypto-Giardia FASTest™ has a reported sensitivity of 97.2% and specificity of 99.9% for the detection of Giardia duodenalis antigen. For the detection of Cryptosporidium parvum antigen, the reported sensitivity is 96.7% and the specificity is 99.9%. According to the results of the FASTest™, 75% (15/20) were negative for Giardia, while use of light microscopy appears to have missed two positive Giardia cases. This can be explained by the fact that Giardia cysts are passed intermittently in the faeces. Faecal samples for microscopy would need to be collected over three consecutive days for each animal. On the other hand, the test strip was able to detect faecal antigen even during the prepatent period.
Conclusions
- In this study, the most prevalent nematode identified was Toxocara canis/cati (6%) and Giardia was the most significant protozoan (4.2%).
- The most popular anthelmintic product was the spot-on, Advocate™ (37.5%). Advocate™ was used in combination with a deworming tablet in 6.25% (3/48) of cases. The remaining 56.25% (27/48) of cases used oral medication only.
- Only 44% (30/68) of known cases were dewormed frequently (two months or less). The remaining 56% were dewormed less frequently than recommended by the manufacturers’ data sheets. The main reason for this infrequency was unwillingness to treat without clinical evidence of infestation.
- The Crypto-Giardia FASTest™ strip was able to detect more positive cases than standard light microscopy. This may be due to the fact that the Giardia cysts would be passed only intermittently in the faeces, while the presence of Giardia antigen would be detected by the test strip during the prepatent period and even if the cysts were not being shed.
- The study highlighted the importance of regular faecal analysis prior to treatment to ensure that all endoparasites identified are covered by the chosen anthelmintic/s.
Contributors
- Allison Mangan, undergraduate MVB student, School of Veterinary Medicine, University College Dublin.
- Amanda Lawlor BAgrSc, senior technical officer, School of Veterinary Medicine, University College Dublin.
- Theo de Waal PhD MRCVS, assistant professor, School of Veterinary Medicine, University College Dublin.
- Sandra Aungier MVM, PhD, assistant professor in veterinary nursing, School of Veterinary Medicine, University College Dublin.
Acknowledgements
The author and contributors thank Joe Cassidy, Brian Cloaks, and Alex Fawcett from the Veterinary Pathobiology Section, and also the staff of the School of Veterinary Medicine, UCD for providing animal faecal samples for this study. We would also like to thank Colm Browne from the Dublin Society for the Prevention of Cruelty to Animals animal shelter, the Kildare Animal Foundation, and veterinary practices throughout Ireland for facilitating us by providing animal stool samples for the study. The study was funded by the Summer Research Project Fund (School of Veterinary Medicine, UCD).
- Roddie G, Stafford P, Holland C, Wolfe A. Contamination of dog hair with eggs of Toxocara canis. Vet Parasitol. 2008;152(1):85-93.
- Hinney B, Gottwald M, Moser J, Reicher B, Schafer BJ, Schaper R, et al. Examination of anonymous canine faecal samples provides data on endoparasite prevalence rates in dogs for comparative studies. Vet Parasitol. 2017;245:106-15.
- Taylor M, Coop R, Wall R. Veterinary Parasitology. 4th ed: Blackwell Publishing; 2016.
- Good B, Holland CV, Taylor MRH, O'Regan M, Larragy J, Moriarty P. Ocular toxocariasis in schoolchildren. Clin Infect Dis. 2004;39(2):173-8.
- Centers for Disease Control and Prevention. Parasites- Toxocariasis 2013 [Available from: https://www.cdc.gov/parasites/toxocariasis/gen_info/faqs.html.
- ESCCAP. GL1: Worm Control in Dogs and Cats 2017 [Available from: https://www.esccap.org/page/GL1+Worm+Control+in+Dogs+and+Cats/25/#.XYNVvyB7mUk.
- Mark-Carew MP, Adesiyun AA, Basu A, Georges KA, Pierre T, Tilitz S, et al. Characterization of Giardia duodenalis infections in dogs in Trinidad and Tobago. Vet Parasitol. 2013;196(1-2):199-202.
- Ryan U, Cacciò SM. Zoonotic potential of Giardia. Int J Parasitol. 2013;43(12–13):943-56.
- Monis PT, Thompson RC. Cryptosporidium and Giardia-zoonoses: fact or fiction? Infect Genet Evol. 2003;3(4):233-44.
- HPRA. Veterinary Medicines Information 2019 [Available from: https://www.hpra.ie/homepage/veterinary.